Cyclic Ugi PPI Library
ChemDiv’s library of small molecule compounds synthesized through the Cyclic Ugi reaction that target protein-protein interaction comprises 9,487 entries
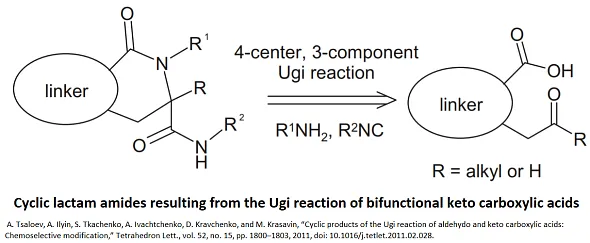
The Cyclic Ugi Protein-Protein Interaction (PPI) Library consists of compounds synthesized through the Cyclic Ugi reaction, a chemical methodology that allows for the rapid generation of diverse and complex cyclic peptides and peptidomimetics. This library is specifically designed to target and modulate protein-protein interactions, which are crucial in many biological processes and have been identified as significant therapeutic targets for various diseases, including cancer, neurodegenerative disorders, and infectious diseases.
Compounds in the Cyclic Ugi PPI Library are characterized by their cyclic structure, which is achieved by the Ugi four-component reaction (Ugi-4CR) followed by intramolecular cyclization. This structure is particularly advantageous for interacting with the extensive and flat surfaces typical of protein-protein interaction sites, offering a high degree of specificity and affinity. The cyclic nature of these compounds enhances their stability, bioavailability, and resistance to proteolytic enzymes, making them more suitable as therapeutic agents compared to their linear counterparts.
The library encompasses a wide range of chemical diversity, incorporating various amino acids, aldehydes, isocyanides, and carboxylic acids in the Ugi reaction to generate a multitude of cyclic compounds. This diversity allows for the exploration of numerous PPI targets, with the potential to identify novel inhibitors or stabilizers of protein-protein interactions. Through the strategic design and synthesis of these compounds, the Cyclic Ugi PPI Library offers a valuable tool for drug discovery efforts aimed at modulating complex biological pathways involved in disease pathogenesis.
Cyclic Ugi PPI compounds play a crucial role in drug discovery by offering a novel approach to modulating protein-protein interactions, which are central to numerous cellular processes and disease mechanisms. These compounds, derived from the innovative Cyclic Ugi chemical reaction, are designed to mimic or disrupt the interface of protein-protein interactions, offering a targeted strategy to influence biological pathways that are often deemed "undruggable" by conventional small molecule drugs. The ability of these cyclic compounds to specifically interact with the complex surfaces of protein-protein interaction sites allows for the precise modulation of critical cellular signals and functions, making them highly attractive candidates for therapeutic development.
The benefits of the Cyclic Ugi PPI small molecule library are manifold. Firstly, the structural diversity and complexity of the library, enabled by the versatile Ugi synthesis, allow for the exploration of a vast chemical space, increasing the likelihood of identifying potent and selective modulators of PPIs. Secondly, the cyclic nature of these compounds confers superior stability, bioavailability, and resistance to enzymatic degradation compared to linear peptides, enhancing their therapeutic potential. Thirdly, the specificity of these molecules for their target PPIs can lead to fewer off-target effects and reduced toxicity, which is a significant advantage in drug development. Additionally, the Cyclic Ugi PPI library's potential to generate novel leads against challenging targets opens up new avenues for the treatment of diseases with unmet medical needs, including various cancers, neurodegenerative diseases, and infectious diseases. Overall, the Cyclic Ugi PPI small molecule library represents a promising and innovative tool in the quest for new and effective therapeutic agents.