Active Biotech (Nasdaq Stockholm: ACTI) today announced initial results from the Phase II proof of concept study of laquinimod in Primary Progressive MS (PPMS) sponsored by Active Biotech's partner Teva Pharmaceuticals Industries Ltd. The primary endpoint of brain atrophy as defined by percent brain volume change (PBVC) from baseline to week 48, was not met after daily oral doses with 0.6 mg laquinimod.
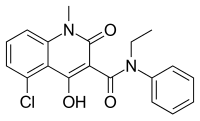
The secondary endpoint of time to confirmed disability progression was also not met. There was, however, a reduction in new T2 lesions observed in patients treated with laquinimod 0.6 mg.
The clinical safety profile of laquinimod 0.6 mg daily in PPMS patients resembled the safety profile demonstrated in relapsing remitting MS patients. The most common adverse events reported by patients treated with laquinimod 0.6 mg daily were nasopharyngities, headache, upper respiratory tract infection and back pain.
Data from the trial will be presented at a future scientific conference and the full results will be published.
Lund, December 01, 2017
http://www.activebiotech.com/



