U.S. Food and Drug Administration Approves Bristol Myers Squibb’s ZEPOSIA® (ozanimod), a New Oral Treatment for Relapsing Forms of Multiple Sclerosis
PRINCETON, NJ, USA - March 26, 2020 - Bristol-Myers Squibb Company (NYSE: BMY) today announced that the U.S. Food and Drug Administration (FDA) approved ZEPOSIA® (ozanimod) 0.92 mg for the treatment of adults with relapsing forms of multiple sclerosis (RMS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.
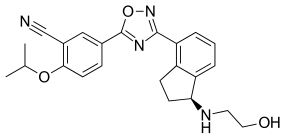
ZEPOSIA, an oral medication taken once daily, is the only approved sphingosine-1-phosphate (S1P) receptor modulator that offers RMS patients an initiation with no genetic test and no label-based first-dose observation required for patients. An up-titration scheme should be used to reach the maintenance dosage of ZEPOSIA, as a transient decrease in heart rate and atrioventricular conduction delays may occur.
Multiple sclerosis (MS) is a disease in which the immune system attacks the protective myelin sheath that covers the nerves, creating damaging lesions that make it harder for signals to travel between each nerve cell. This “signal breakdown” can lead to symptoms and relapses.
“With the FDA approval of ZEPOSIA, appropriate patients with relapsing forms of multiple sclerosis will have another oral treatment option with meaningful efficacy to help address the disease’s hallmark relapses and brain lesions," said Samit Hirawat, M.D., chief medical officer, Bristol Myers Squibb. “ZEPOSIA has substantial clinical potential, and we are well positioned with our heritage in transformational science to ensure this innovative compound ultimately benefits as many patients as possible.”
The approval is based on data from the largest pivotal, head-to-head RMS studies with an active comparator to date: the randomized, active-controlled Phase 3 SUNBEAM™ (safety and efficacy of ZEPOSIA versus interferon beta-1a in relapsing multiple sclerosis) and RADIANCE™ (safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ZEPOSIA in relapsing multiple sclerosis) Part B clinical trials of more than 2,600 adults. In both trials – as compared to AVONEX® (interferon beta-1a), ZEPOSIA delivered powerful efficacy as measured by annualized relapse rate (ARR), as well as on the number and size of brain lesions.
26 March 2020
https://pipelinereview.com/



