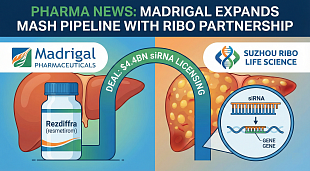
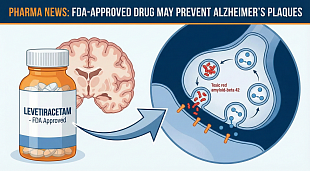
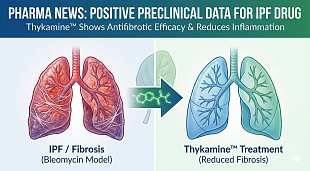
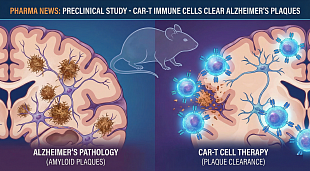
EnGeneIC initiates Phase I trial of nanocellular anti-Covid-19 vaccine
EnGeneIC has initiated dosing in Phase I clinical trial of its first-ever nanocellular technology-based anti-Covid-19 vaccine in healthy adults.
Named EnGeneIC Dream Vector (EDV), the nanocell technology platform was demonstrated to induce a wide-ranging and robust anti-Covid-19 immune response in pre-clinical animal studies.
It also showed the ability to neutralise the known mutant SARS-CoV-2 viruses of concern, especially the Delta variant, EnGeneIC noted.
The EDVs comprise three molecules, one that generates the SARS-CoV-2 Spike protein in the EDV nanocell and a second molecule that concurrently induces the activation of vital cells of the immune system associated with virus-fighting.
The third molecule can alter the anti-virus antibody response into increased affinity velcro-like antibodies that can kill the mutant viruses.
To date, two subjects in the Phase I trial were dosed at the St Vincent’s Hospital in Melbourne, Australia. The trial is intended to assess the vaccine’s safety and immune responses against Covid-19.
Immune readouts are anticipated to be reported at one and three months after dosing, the company noted.
EnGeneIC co-CEOs Dr Jennifer MacDiarmid and Dr Himanshu Brahmbhatt said: “We appreciate that the vaccine rollout is ongoing in Australia, but no vaccine to date is directed towards immune-compromised people and these people were excluded from vaccine trials aimed at obtaining emergency approval.
09 Sep 2021
https://www.pharmaceutical-technology.com/