Exelixis Announces U.S. FDA Approval of CABOMETYX® (cabozantinib) in Combination with OPDIVO® (nivolumab) as a First-Line Treatment for Patients with Advanced Renal Cell Carcinoma
ALAMEDA, CA, USA I January 22, 2021 I Exelixis, Inc. (NASDAQ: EXEL) today announced that the U.S. Food and Drug Administration (FDA) approved CABOMETYX® (cabozantinib) for patients with advanced renal cell carcinoma (RCC) as a first-line treatment in combination with OPDIVO® (nivolumab). RCC is the most common form of kidney cancer, which is among the 10 most frequently diagnosed cancers in the U.S. annually.
“This combination of cabozantinib and nivolumab significantly improved key efficacy measures compared to sunitinib – progression-free survival, overall survival and objective response rate – while showing a low rate of treatment discontinuations due to side effects. The therapeutic benefit demonstrated in CheckMate -9ER and quality of life measures explored emphasize the role of this combination for patients with advanced kidney cancer,” said Dr. Toni Choueiri, Director of the Lank Center for Genitourinary Oncology at Dana-Farber Cancer Institute and the Jerome and Nancy Kohlberg Professor of Medicine at Harvard Medical School. “With this important FDA approval, the combination is poised to become a standard in newly diagnosed metastatic kidney cancer.”
The approval is based on results from CheckMate -9ER, a phase 3 pivotal trial evaluating the combination of CABOMETYX and OPDIVO compared with sunitinib in previously untreated advanced or metastatic RCC. These results were presented during the European Society of Medical Oncology Virtual Congress 2020 in September. The FDA reviewed the application for CABOMETYX and OPDIVO under the Real-Time Oncology Review (RTOR) pilot program and Fast Track designation. The RTOR pilot program, which allows an applicant to pre-submit components of the application to allow the FDA to review clinical trial data before the complete filing is submitted, aims to explore a more efficient review process to ensure safe and effective treatments are available to patients sooner.
“As the only combination treatment regimen to double median progression-free survival and objective response rate compared with sunitinib while also significantly improving overall survival, we are excited that CABOMETYX in combination with OPDIVO is now available for the first-line treatment of patients with advanced kidney cancer,” said Michael M. Morrissey, Ph.D., President and Chief Executive Officer of Exelixis. “This approval is a meaningful milestone for this patient community and speaks to the broad potential of CABOMETYX as we continue to generate important clinical trial results supporting its use in combination with immune checkpoint inhibitors to benefit patients with other difficult-to-treat cancers. We would like to thank the clinical trial participants, the physicians and their staff who participated in the CheckMate -9ER trial and to acknowledge the team at the FDA for their collaboration during the review of our application.”
In CheckMate -9ER, the combination regimen significantly improved overall survival (OS) compared with sunitinib (HR= 0.60, 98.89% CI 0.40-0.89; p=0.001). Median OS has not yet been reached in either treatment arm. Median progression-free survival (PFS) was doubled at 16.6 months for CABOMETYX in combination with OPDIVO compared with 8.3 months for sunitinib (HR 0.51, 95% CI 0.41-0.64; p<0.0001). Objective response rate (ORR) was also doubled: 56% with CABOMETYX in combination with OPDIVO and 27% with sunitinib (p<0.0001). Consistent results for PFS were observed across subgroups of International Metastatic RCC Database Consortium risk status and PD-L1 tumor expression with CABOMETYX in combination with OPDIVO.
CABOMETYX in combination with OPDIVO was generally well tolerated and reflected the known safety profiles of the tyrosine kinase inhibitor and immunotherapy components in previously untreated advanced RCC. The most common adverse reactions reported in at least 20% of patients treated with CABOMETYX in combination with OPDIVO were diarrhea, fatigue, hepatotoxicity, palmar-plantar erythrodysesthesia, stomatitis, rash, hypertension, hypothyroidism, musculoskeletal pain, decreased appetite, nausea, dysgeusia, abdominal pain, cough and upper respiratory tract infection.
Exelixis’ partner Ipsen Pharma SAS (Ipsen), which has exclusive rights to commercialize and develop CABOMETYX outside of the U.S. and Japan, and Bristol-Myers Squibb Company (BMS) each submitted type II variation applications for CABOMETYX in combination with OPDIVO to the European Medicines Agency (EMA). On September 12, 2020, the EMA validated the type II variations, confirming the submissions are complete and beginning the EMA’s centralized review process. On October 27, 2020 Takeda Pharmaceutical Company Limited (Takeda), Exelixis’ partner responsible for the clinical development and commercialization of CABOMETYX in Japan, and Ono Pharmaceuticals Co., Ltd., BMS’ development and commercialization partner in Japan, submitted a supplemental application to the Japanese Ministry of Health, Labour and Welfare for manufacturing and marketing approval of CABOMETYX in combination with OPDIVO for the treatment of patients with unresectable, advanced or metastatic RCC.
About CABOMETYX® (cabozantinib)
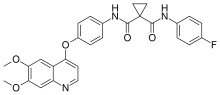
In the U.S., CABOMETYX tablets are approved for the treatment of patients with advanced RCC; for the treatment of patients with HCC who have been previously treated with sorafenib; and for patients with advanced RCC as a first-line treatment in combination with OPDIVO (nivolumab). CABOMETYX tablets have also received regulatory approvals in the European Union and additional countries and regions worldwide. In 2016, Exelixis granted Ipsen exclusive rights for the commercialization and further clinical development of cabozantinib outside of the United States and Japan. In 2017, Exelixis granted exclusive rights to Takeda Pharmaceutical Company Limited for the commercialization and further clinical development of cabozantinib for all future indications in Japan. Exelixis holds the exclusive rights to develop and commercialize cabozantinib in the United States.
Published on Sunday, 24 January 2021
https://pipelinereview.com/