FDA approves Bristol Myers’ Zeposia for UC
The US Food and Drug Administration yesterday approved Bristol Myers Squibb’s (NYSE: BMY) Zeposia (ozanimod) 0.92mg for the treatment of adults with moderately to severely active ulcerative colitis (UC), a chronic inflammatory bowel disease (IBD).
Zeposia, an oral medication taken once daily, is the first and only sphingosine 1-phosphate (S1P) receptor modulator approved for patients with moderately to severely active UC. It will compete with Takeda’s (TYO: 4502) Entyvio (vedolizumab), which was approved for UC by the FDA in 2014.
Sales forecast
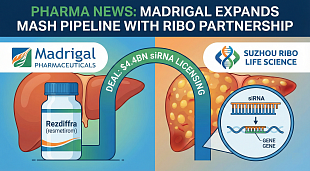
Bristol Myers gained the drug, approved last year for treating multiple sclerosis patients, in 2019 through its $74 billion buyout of Celgene. In a recent client noted, Cowen analyst Steve Scala estimated Zeposia sales of $150 million in 2021, $400 million in 2022 and $1 billion in 2025.
“Despite the availability of approved therapies, there is still unmet need and an opportunity to deliver additional treatment options to help patients better manage their disease,” said Adam Lenkowsky, general manager and head, US, cardiovascular, immunology and oncology, Bristol Myers Squibb.
The approval is based on data from True North, a pivotal Phase III trial evaluating Zeposia as an induction and maintenance therapy versus placebo in adult patients with moderately to severely active UC.
Access Support program
Bristol Myers said it is committed to making Zeposia accessible to the patients who need it. The Zeposia 360 Support program will facilitate access for appropriate patients with UC. This includes a co-pay program for eligible appropriate patients to pay as little as $0 for their Zeposia prescription, assistance with financial support and a program that may help eligible patients with commercial insurance to receive free medication while they are experiencing delays or issues with insurance coverage. Support for eligible patients is also available for the routine initiation assessments, such as lab work.
A Marketing Authorization Application for Zeposia for the treatment of adults with moderately to severely active UC in the European Union is currently under review with the European Medicines Agency (EMA). A regulatory decision from the EMA is expected the second half of 2021.
29-05-2021
https://www.thepharmaletter.com/