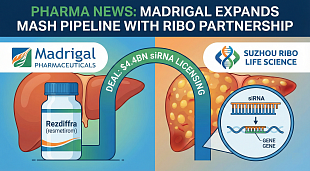
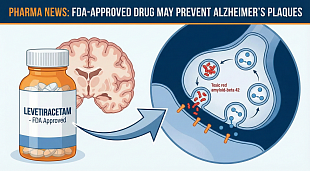
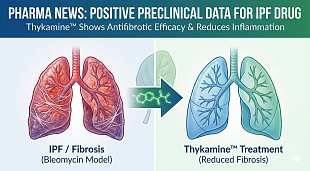
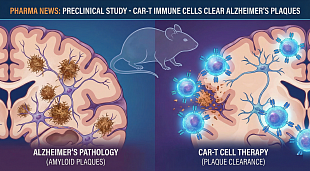
Narsoplimab FDA Approval Status
Narsoplimab is an investigational human monoclonal antibody in development for the treatment of hematopoietic stem cell transplant-associated thrombotic microangiopathy (HSCT-TMA).
Hematopoietic stem cell transplant-associated thrombotic microangiopathy (HSCT-TMA) is a significant and often lethal complication of stem cell transplantation. HSCT-TMA is a systemic, multifactorial disorder caused by endothelial cell damage induced by conditioning regimens, immunosuppressant therapies, infection, graft-versus-host disease, and other factors associated with stem cell transplantation.
Narsoplimab works by targeting mannan-binding lectin-associated serine protease-2 (MASP-2), a novel pro-inflammatory protein target and the effector enzyme of the lectin pathway of complement.
A biologics license application (BLA) has been submitted to the U.S. FDA for use of narsoplimab in the treatment of hematopoietic stem cell transplant-associated thrombotic microangiopathy (HSCT-TMA), and the drug is in Phase 3 clinical programs for immunoglobulin A (IgA) nephropathy and atypical hemolytic uremic syndrome (aHUS).
The FDA has granted narsoplimab breakthrough therapy designations for HSCT-TMA and for IgA nephropathy; orphan drug status for the prevention (inhibition) of complement-mediated thrombotic microangiopathies, for the treatment of HSCT-TMA and for the treatment of IgA nephropathy; and fast track designation for the treatment of patients with atypical hemolytic uremic syndrome (aHUS).
Oct 3, 2021
https://www.drugs.com/