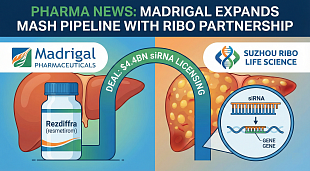
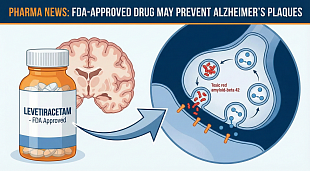
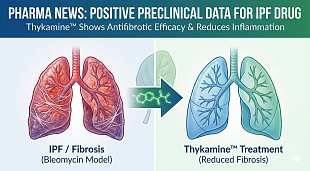
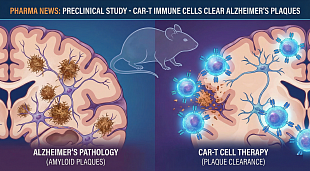
NuCana Receives Fast Track Designation from the U.S. Food and Drug Administration for Acelarin® (NUC-1031) for the Treatment of Biliary Tract Cancer
EDINBURGH, United Kingdom, Sept. 29, 2021 (GLOBE NEWSWIRE) -- NuCana plc (NASDAQ: NCNA) announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to Acelarin (NUC-1031), currently being evaluated in a Phase III study (NuTide:121) for the first-line treatment of patients with advanced biliary tract cancer. Fast Track is a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and address an unmet medical need.
“We are very pleased that the FDA recognizes the potential of Acelarin to address the significant unmet need of patients with biliary tract cancer,” said Hugh S. Griffith, NuCana’s Founder and Chief Executive Officer. “We recently announced enrollment of 418 evaluable patients in our Phase III study, which is expected to enable the first interim analysis in the first half of 2022. This has the potential to allow for an accelerated approval of a new drug application (NDA) for Acelarin in the United States. With both Fast Track and Orphan Drug designations in place, we look forward to working closely with the FDA in our efforts to gain approval for Acelarin as the first approved front-line treatment option for patients with biliary tract cancer.”
About Fast Track Designation
Fast track is a process designed to facilitate the development, and expedite the review of drugs to treat serious conditions and fill an unmet medical need. The purpose is to get important new drugs to the patient earlier. Filling an unmet medical need is defined as providing a therapy where none exists or providing a therapy which may be potentially better than available therapy. Once a drug receives Fast Track designation, early and frequent communication between the FDA and a drug company is encouraged throughout the entire drug development and review process. The frequency of communication assures that questions and issues are resolved quickly, often leading to earlier drug approval and access by patients.
About Biliary Tract Cancer
Biliary tract cancer, including cholangiocarcinoma, gallbladder and ampullary carcinoma, are a group of cancers originating in the biliary tract. The biliary tract is comprised of the gallbladder and interconnecting ducts responsible for the transport of bile from the liver to the gallbladder and small intestine. Approximately 178,000 new cases of biliary tract cancer are diagnosed each year worldwide, with more than 18,000 of those diagnoses in the United States. There are currently no agents approved for the first-line treatment of patients with advanced biliary tract cancer; however, the worldwide standard of care in these patients is the combination of gemcitabine and cisplatin. Patients receiving this regimen have a median overall survival of 11.7 months.
About NuCana
NuCana is a clinical-stage biopharmaceutical company focused on significantly improving treatment outcomes for patients with cancer by applying our ProTide technology to transform some of the most widely prescribed chemotherapy agents, nucleoside analogs, into more effective and safer medicines. While these conventional agents remain part of the standard of care for the treatment of many solid and hematological tumors, their efficacy is limited by cancer cell resistance mechanisms and they are often poorly tolerated. Utilizing our proprietary technology, we are developing new medicines, ProTides, designed to overcome key cancer resistance mechanisms and generate much higher concentrations of anti-cancer metabolites in cancer cells. NuCana’s robust pipeline includes three ProTides in clinical development. Acelarin (NUC-1031) and NUC-3373, are new chemical entities derived from the nucleoside analogs gemcitabine and 5-fluorouracil, respectively, two widely used chemotherapy agents. Acelarin is in a Phase 3 study for patients with advanced biliary tract cancer. NUC-3373 is in a Phase 1b/2 study in patients with metastatic colorectal cancer. Our third ProTide, NUC-7738, is a transformation of a novel anti-cancer nucleoside analog (3’-deoxyadenosine) and is in a Phase 1 study for patients with advanced solid tumors.
Sep 29, 2021
https://ir.nucana.com/i