BioXcel’s Schizophrenia and Bipolar-Associated Agitation Drug Approved
BioXcel Therapeutics has gained the FDA’s approval for Igalmi, its sublingual film formulation of dexmedetomidine, for the treatment of agitation associated with schizophrenia or bipolar disorder.
The drug is a sedative that is commonly administered by continuous infusion during surgical procedures.
The approval was based on data from two phase 3 trials in which participants given the sublingual treatment in 120-mg or 180-mg doses showed a significant decrease in agitation.
All adverse events in the two trials were mild to moderate, but the drug’s possible side effects include low blood pressure, a slowed heart rate and drowsiness.
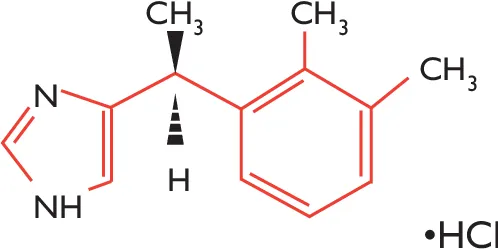
Dexmedetomidine, sold under the trade name Precedex among others, is an anxiolytic, sedative, and pain medication. Dexmedetomidine is notable for its ability to provide sedation without risk of respiratory depression (unlike other commonly used drugs such as propofol and fentanyl) and can provide cooperative or semi-rousable sedation. Similar to clonidine, it is a sympatholytic drug that acts as an agonist of α2-adrenergic receptors in certain parts of the brain.
About BioXcel Therapeutics, Inc.
BioXcel Therapeutics, Inc. is a biopharmaceutical company utilizing artificial intelligence approaches to develop transformative medicines in neuroscience and immuno-oncology. The Company’s drug re-innovation approach leverages existing approved drugs and/or clinically validated product candidates together with big data and proprietary machine learning algorithms to identify new therapeutic indices. The Company’s commercial product, IGALMI (developed as BXCL501) is a proprietary, sublingual film formulation of dexmedetomidine approved by the FDA for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder in adults. BXCL501 is also being evaluated for the acute treatment of Alzheimer’s disease, and as an adjunctive treatment for major depressive disorder. The company is also developing BXCL701, an investigational, orally administered, systemic innate immunity activator for the treatment of aggressive forms of prostate cancer and advanced solid tumors that are refractory or treatment naïve to checkpoint inhibitors.
April 8, 2022



