EU Approves AstraZeneca’s Lupus Drug Saphnelo
The European Union has approved AstraZeneca’s lupus drug Saphnelo (anifrolumab), a first-in-class type 1 interferon receptor antibody as an add-on therapy for the treatment of severe, active autoantibody-positive systemic lupus erythematosus, despite receiving standard therapy.
Saphnelo will now begin competing with GlaxoSmithKline’s Benlysta (belimumab) for the treatment of moderate-to-severe systemic lupus erythematosus.
Saphnelo met primary endpoints in recent phase 2 and 3 trials, although it missed key endpoints in a second phase 3 trial, the company said.
The drug was recently approved in the U.S., Japan and Canada, and is undergoing review in several other countries.
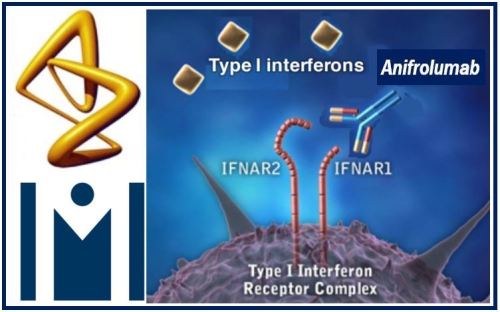
Saphnelo
Saphnelo (anifrolumab) is a fully human monoclonal antibody that binds to subunit 1 of the type I IFN receptor, blocking the activity of type I IFN. Type I IFNs, such as IFN-alpha, IFN-beta and IFN-kappa, are cytokines involved in regulating the inflammatory pathways implicated in SLE.The majority of adults with SLE have increased type I IFN signalling, which is associated with increased disease activity and severity.
AstraZeneca in Respiratory & Immunology
Respiratory & Immunology, part of BioPharmaceuticals, is one of AstraZeneca’s main disease areas and is a key growth driver for the Company.
AstraZeneca is an established leader in respiratory care with a 50-year heritage. The Company aims to transform the treatment of asthma and chronic obstructive pulmonary disease (COPD) by focusing on earlier biology-led treatment, eliminating preventable asthma attacks and removing COPD as a top-three leading cause of death. The Company’s early respiratory research is focused on emerging science involving immune mechanisms, lung damage and abnormal cell-repair processes in disease and neuronal dysfunction.
With common pathways and underlying disease drivers across respiratory and immunology, AstraZeneca is following the science from chronic lung diseases to immunology-driven disease areas. The Company’s growing presence in immunology is focused on five mid- to late-stage franchises with multi-disease potential, in areas including rheumatology (including SLE), dermatology, gastroenterology, and systemic eosinophilic-driven diseases. AstraZeneca’s ambition in Respiratory & Immunology is to achieve disease modification and durable remission for millions of patients worldwide.
February 17, 2022



