FDA Approves Higher Dose of Novo Nordisk’s Type 2 Diabetes Drug
The FDA has approved a 2-mg dose of Novo Nordisk’s type 2 diabetes drug Ozempic (semaglutide), a once-weekly glucagon-like peptide-1 (GLP-1) analogue that is now available in 0.5-mg, 1-mg and 2-mg doses.
The approval was based on positive results from a 961-participant phase 3 trial. Patients with an average three-month blood sugar level of 8.9 percent achieved a reduction in blood sugar of 2.1 percentage points compared with 1.9 percent in patients given 1 mg of Ozempic, the company said.
The higher dose will help individuals with a higher blood sugar count who have been unable to meet their target, the company said. The American Diabetes Association’s target blood sugar level is below 7 percent.
The 2-mg Ozempic dose was already approved in the EU, Canada and Switzerland.
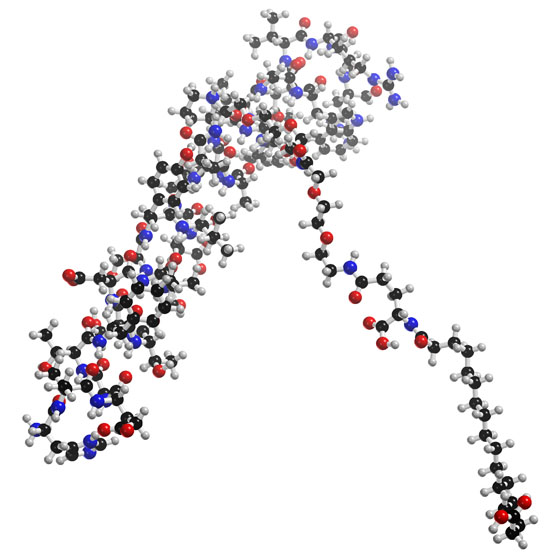
Semaglutide, sold under the brand name Ozempic among others, is an antidiabetic medication used for the treatment of type 2 diabetes and long-term weight management. Semaglutide acts like human glucagon-like peptide-1 (GLP-1) in that it increases insulin secretion, thereby increasing sugar metabolism. It is distributed as a metered subcutaneous injection in a prefilled pen, or as an oral form. One of its advantages over other antidiabetic drugs is that it has a long duration of action, so a once-a-week injection is sufficient.
March 30, 2022