Newly discovered chemical reactions may explain the origin of life
Exactly how inanimate molecules came to life is one of science’s most enigmatic mysteries. Now Scripps Research scientists have discovered a new set of chemical reactions that can produce the building blocks of life from materials thought to be common in the primeval soup of early Earth. The first forms of life are believed to have appeared on Earth billions of years ago from a nutrient-rich mixture often referred to as “primal soup”. Basically, the molecules in it started reacting with each other due to additional energy, such as lightning strikes or hydrothermal vents, until they formed basic organic compounds, then amino acids, which can then combine into peptides and proteins, and finally into life cells.
This is obviously a huge oversimplification and the specific chemical reactions that took place during the process remain unclear. The scientists conducted their research by making their own versions of the original soup, based on what was considered hearty at the time, and exposing it to various conditions to see what happened and how easily precursors of life could form.
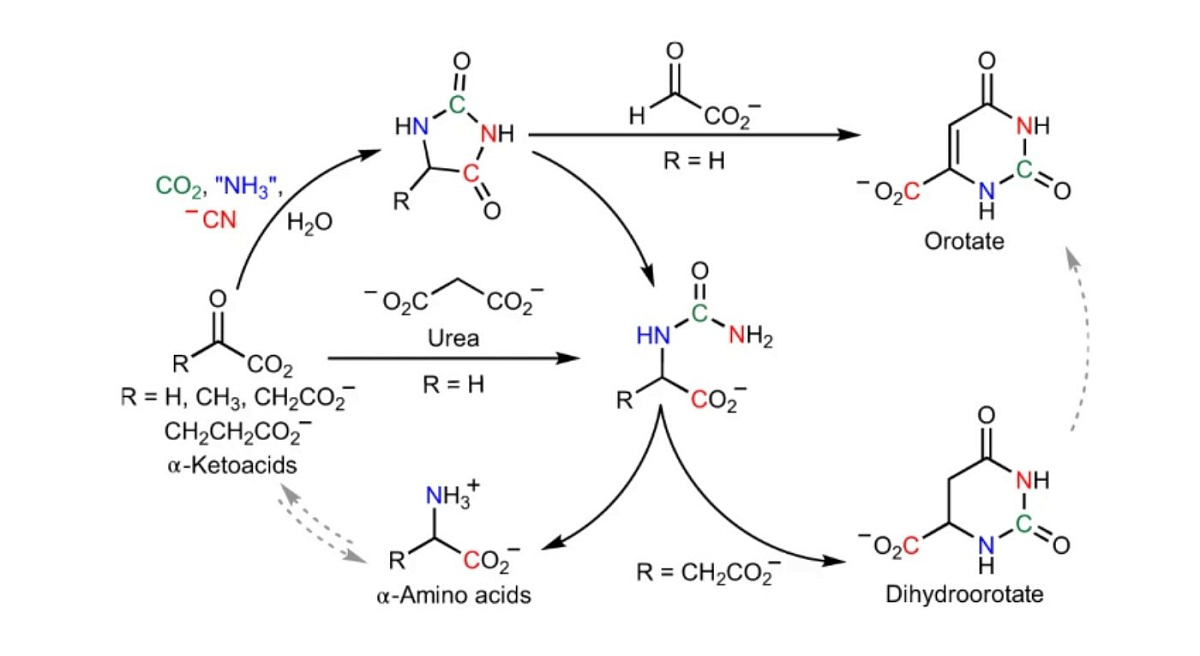
In this new study, Scripps scientists tweaked their original soup recipe and discovered a new set of chemical reactions using relatively simple ingredients that were likely common on Earth. With just cyanide, ammonia, carbon dioxide, and alpha-keto acids, the team began to see the soup producing amino acids.
Each of the four ingredients has a role to play. Alpha-keto acids are the precursors that living cells use today to make amino acids. Ammonia is the source of nitrogen needed for the conversion process. Cyanide does the conversion and carbon dioxide accelerates it all the time.
We expected this to be difficult to understand, and it turned out to be even easier than we imagined. Said Ramanarayanan Krishnamurthy, lead author of the study. If you just mix keto acid, cyanide, and ammonia, it will stay there. As soon as you add carbon dioxide, even in trace amounts, the reaction speeds up.
The team says the process is essentially how amino acids are made in living cells, except the cyanide is a substitute for enzymes that didn’t yet exist in the original soup. This simplicity and similarity to current biological processes suggests that it is a more likely source of early life than other hypotheses that require radically different chemistry.
Analysis of the chemical soup revealed that orotate, which is a precursor to the nucleotides that make up DNA and RNA, is also a byproduct of this process, suggesting that a multitude of components of life could be produced in this way.
We want to continue the research to see what kind of chemistry can emerge from this mixture Krishnamurthy said. Can amino acids start making small proteins? Could one of these proteins come back and start acting like an enzyme to produce more of these amino acids?
The research results have been published in the journal natural chemistry.
Prebiotic synthesis of α-amino acids and orotate from α-keto acids enhances transition to existing metabolic pathways | photo: Nature Chemistry
08/08/2022



