Aptevo Therapeutics Provides Program Update for Bispecific APVO711 ( PD-L1 х CD40)
As lead candidate mipletamig continues to outperform efficacy and safety benchmarks in AML trials, APVO711 exemplifies emerging innovation from Aptevo's proprietary ADAPTIR platform. In preclinical studies, APVO711 demonstrates dual anti-cancer functionality with broad solid tumor potential and developability
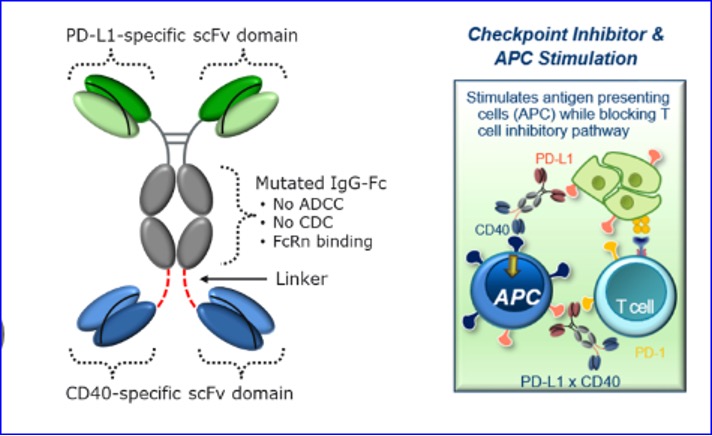
Aptevo Therapeutics Inc., a clinical-stage biotechnology company focused on developing novel immune-oncology therapeutics based on its proprietary ADAPTIR® and ADAPTIR-FLEX® platform technologies, today provided a program update for APVO711, a dual mechanism bispecific utilizing the PD-L1 arm to block the PD-1/PD-L1 pathway and the CD40 arm to enhance T cell priming through activation of the stimulatory receptor CD40 on antigen presenting cells. This update highlights the depth and scientific versatility of Aptevo's existing pipeline and reinforces the Company's ability to advance differentiated candidates for solid tumors with significant unmet need. Currently in preclinical development, APVO711 reflects the potential of Aptevo's platform-based strategy to generate bispecific therapeutics with novel mechanisms and broad clinical relevance.
"With APVO711, we're advancing an innovative dual-mechanism approach to immunotherapy-one that not only boosts the immune system through PD-L1 blockade but also facilitates T-cell priming via CD40 activation. This is important in the development of new therapeutics for cancers that have proven resistant to standard checkpoint therapies. We believe APVO711 has the potential to unlock deeper, more sustained responses, and ultimately expand the reach of immunotherapy to patients who need it most," said Marvin White, President and CEO of Aptevo.
About APVO711
Novel Mechanism: APVO711 is a bispecific antibody targeting PD-L1 × CD40-combining checkpoint inhibition with immune activation in a single molecule.
Designed for Potency: Unlike traditional checkpoint inhibitors, APVO711 simultaneously blocks immune suppression and primes antigen presenting cells to trigger robust T-cell responses.
Targeting the Unmet Need: Developed to address a wide variety of tumor types and augment the anti-tumor responses achieved with PD-1/PD-L1 blockade alone.
Pipeline Synergy: Represents a key part of Aptevo's novel immuno-oncology platform, with strong potential for combination strategies across solid tumors.
Momentum Building: Preclinical studies demonstrate dual anti-cancer functionality.
"APVO711 represents a novel immunotherapy approach - by targeting both PD-L1 and CD40, this bispecific candidate is designed to block tumor-driven immune suppression while simultaneously priming T cells for a stronger, more durable anti-tumor response. In a landscape where many patients fail to respond to checkpoint inhibitors alone, APVO711 offers a rational strategy to expand the benefits of immunotherapy to a broader range of cancers and patients," said Michelle H. Nelson, Ph.D., Director of Immunobiology at Aptevo Therapeutics.
Aptevo Therapeutics Inc., a clinical-stage biotechnology company focused on developing novel immune-oncology therapeutics based on its proprietary ADAPTIR® and ADAPTIR-FLEX® platform technologies, today provided a program update for APVO711, a dual mechanism bispecific utilizing the PD-L1 arm to block the PD-1/PD-L1 pathway and the CD40 arm to enhance T cell priming through activation of the stimulatory receptor CD40 on antigen presenting cells. This update highlights the depth and scientific versatility of Aptevo's existing pipeline and reinforces the Company's ability to advance differentiated candidates for solid tumors with significant unmet need. Currently in preclinical development, APVO711 reflects the potential of Aptevo's platform-based strategy to generate bispecific therapeutics with novel mechanisms and broad clinical relevance.
"With APVO711, we're advancing an innovative dual-mechanism approach to immunotherapy-one that not only boosts the immune system through PD-L1 blockade but also facilitates T-cell priming via CD40 activation. This is important in the development of new therapeutics for cancers that have proven resistant to standard checkpoint therapies. We believe APVO711 has the potential to unlock deeper, more sustained responses, and ultimately expand the reach of immunotherapy to patients who need it most," said Marvin White, President and CEO of Aptevo.
About APVO711
Novel Mechanism: APVO711 is a bispecific antibody targeting PD-L1 × CD40-combining checkpoint inhibition with immune activation in a single molecule.
Designed for Potency: Unlike traditional checkpoint inhibitors, APVO711 simultaneously blocks immune suppression and primes antigen presenting cells to trigger robust T-cell responses.
Targeting the Unmet Need: Developed to address a wide variety of tumor types and augment the anti-tumor responses achieved with PD-1/PD-L1 blockade alone.
Pipeline Synergy: Represents a key part of Aptevo's novel immuno-oncology platform, with strong potential for combination strategies across solid tumors.
Momentum Building: Preclinical studies demonstrate dual anti-cancer functionality.
"APVO711 represents a novel immunotherapy approach - by targeting both PD-L1 and CD40, this bispecific candidate is designed to block tumor-driven immune suppression while simultaneously priming T cells for a stronger, more durable anti-tumor response. In a landscape where many patients fail to respond to checkpoint inhibitors alone, APVO711 offers a rational strategy to expand the benefits of immunotherapy to a broader range of cancers and patients," said Michelle H. Nelson, Ph.D., Director of Immunobiology at Aptevo Therapeutics.