Spinogenix’s ALS hopeful to advance to registrational trial
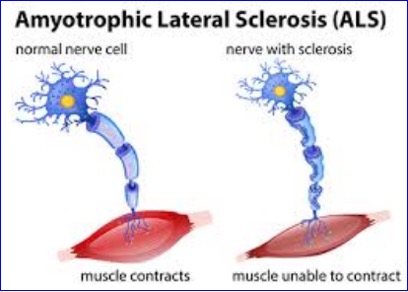
Spinogenix’s first-in-class ALS treatment, SPG302 has been shown to slow disease progression in 82% of patients during a Phase IIa study. Spinogenix is progressing its lead candidate, SPG302, to a registrational trial following the positive outcome of its mid-stage study in amyotrophic lateral sclerosis (ALS).
During the Phase IIa study (NCT05882695), SPG302 met its primary endpoint of safety and tolerability in the ALS population, with no treatment-emergent serious adverse events (SAEs) reported over a six-month period of daily dosing.
The first-in-class drug also triggered a stable or improved rate of ALS-associated decline in 82% of patients at the end of the treatment period. This was defined by each patient’s revised ALS Functional Rating Scale (ALSFRS-R) score.
When compared with historical controls pulled from the PRO-ACT database, patients treated with SPG302 also experienced a 76% slower rate of decline through the six month treatment period. The PRO-ACT database is the largest publicly integrated source of data from ALS clinical trials.
Meanwhile, electroencephalogram (EEG) recordings taken throughout the study revealed “improvements in ALS-associated patterns”, which Spinogenix says supports the drug’s observed capacity to stave off functional decline. Biomarkers also pointed to the drug’s effects on areas of the brain impacted by ALS.
The full results – including further exploratory endpoints explored in the Phase IIa trial – will be debuted in an oral presentation at the International Symposium on ALS/Motor Neurone Disease (MND), which will be held in San Diego, California, US, on 5 December.
Moving forward, the company plans to perform a “registrational-directed” ALS trial, which could see the drug join four targeted therapies that have gained approval in this indication.
This includes Biogen’s antisense oligonucleotide, Qalsody (tofersen), which achieved a conditional approval from the US Food and Drug Administration (FDA) in 2023. This is despite its failure to significantly halt disease progression compared with placebo in the Phase III VALOR study (NCT02623699). The FDA said that the reductions in plasma NfL seen in VALOR were “reasonably likely to predict a clinical benefit in patients”.
BrainStorm Therapeutics’ NurOwn experienced a similar issue, as the cell therapy failed to meet its primary endpoints in its registrational trial after a tumultuous development journey. However, the biotech is taking another shot at the drug’s approval, with the FDA approving a Phase IIIb trial on the drug in May 2025.
Meanwhile, Biogen and Ionis Pharmaceuticals’ BIIB105 scrapped the development of its ALS therapy in 2024 after the drug flopped in the Phase I/II ALSpire study (NCT04494256). These drugs fall into a trend of failures within the ALS space, as the indication’s complex pathophysiology has made it challenging to develop disease-modifying medications.
If SPG302 were to secure regulatory approval, it would enter a global market that GlobalData estimates will be worth $4.3bn by 2031.