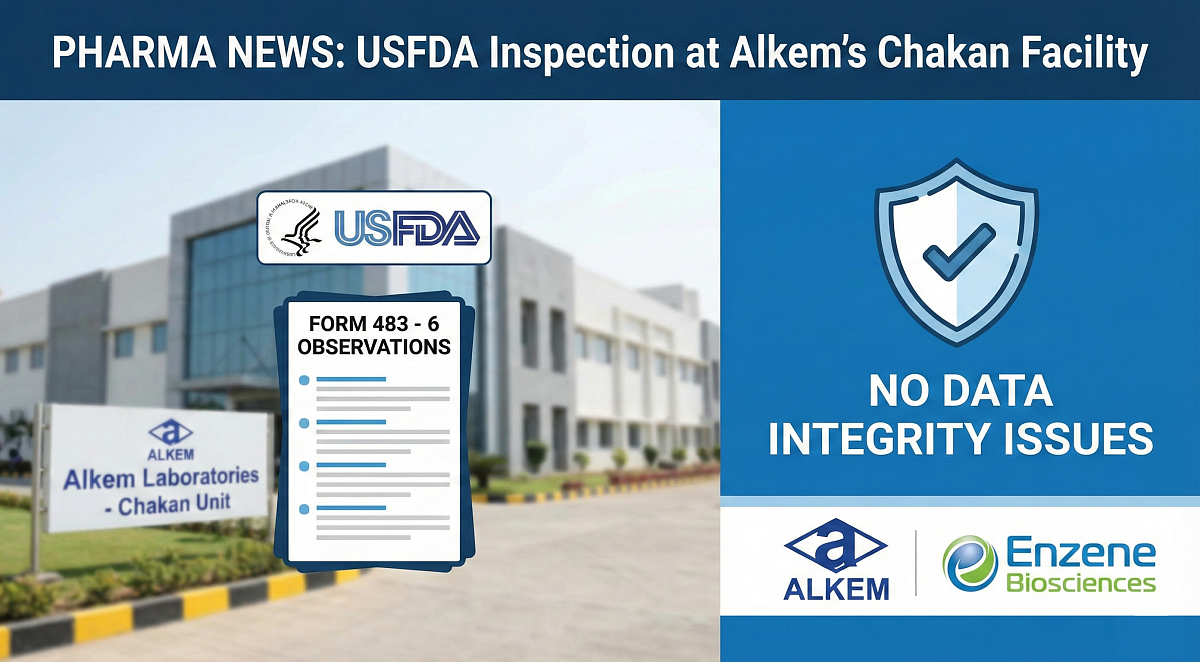
Alkem Laboratories announced that its subsidiary, Enzene Biosciences, has received a Form 483 from the US Food and Drug Administration (USFDA). The inspection at the Chakan manufacturing unit resulted in six procedural observations. Crucially, the company highlighted that there were zero observations related to data integrity.
The USFDA completed a pre-approval inspection on February 13, 2026, at the manufacturing facility of Enzene Biosciences, the drug firm said in a regulatory filing. "At the conclusion of the inspection, the USFDA issued a Form 483 with 6 procedural observations," the company added.
Quality Assurance: The company has achieved zero observations related to data integrity, a critical validation of its quality systems and the reliability of regulatory filings, Alkem stated.
Enzene is currently in the process of preparing and submitting its response to the USFDA within the stipulated timeline and has initiated appropriate corrective and preventive actions.
As per the USFDA, a Form 483 is issued to a firm's management at the conclusion of an inspection when the investigator has observed any conditions that may constitute violations of the Food, Drug and Cosmetic (FD&C) Act and related Acts.
Following the news, Alkem Labs shares on Monday ended 1.37 per cent up at Rs 5,475.55 apiece on the BSE, reflecting market confidence in the resolution of the observations.