Alteogen Touts Monthly Obesity Drug Matching Retatrutide in Preclinical Tests
- Company: Alteogen
- Platform: Ultra–Long-Acting (NexP)
- Dosing: Once-Monthly
- Comparator: Retatrutide (Lilly)
- Result: Similar Weight Loss
- Benefit: Less Yo-Yo Regain
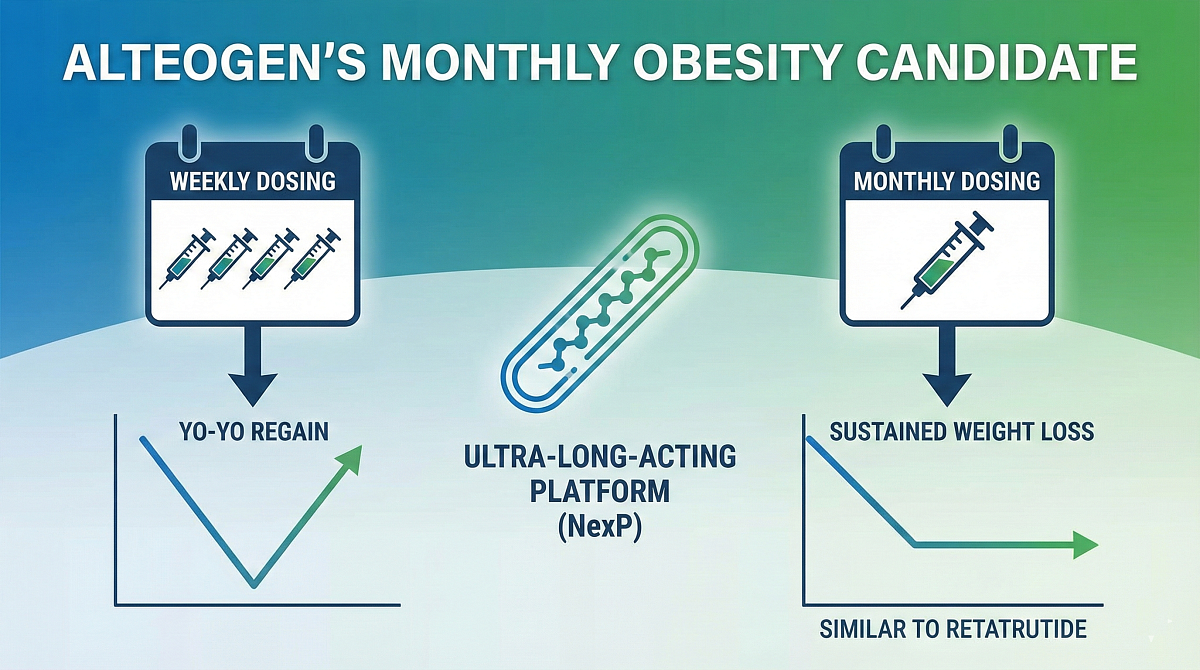
Alteogen said on the 9th that its once-monthly obesity drug candidate, which applies its in-house ultra–long-acting platform, showed not only weight-loss effects in preclinical testing but also a tendency for less so-called yo-yo weight regain after dosing stopped.
Platform Technology: NexP
Alteogen's ultra–long-acting platform extends the dosing interval of its existing long-acting NexP platform and is being developed to shift from a "once-weekly" dosing formulation to "once-monthly" dosing.
In the global anti-obesity drug market, once-weekly dosing has already become the standard, while next-generation candidates are being developed to deliver greater weight loss and improved dosing convenience.
Comparison to Retatrutide and Market Leaders
Among current competitors, retatrutide, a once-weekly subcutaneous injection being developed by Eli Lilly and Company, posted up to 29% weight loss in clinical trials and is drawing attention for higher efficacy compared with "Wegovy" and "Mounjaro," which are currently on the market.
Alteogen's once-monthly obesity drug candidate confirmed a long half-life and sustained drug concentration in animal pharmacokinetics (PK) testing last year.
Preclinical Results: Efficacy and Weight Maintenance
This time, using an obese rat model, the company evaluated weight-loss effects and post-discontinuation weight changes through pharmacodynamics (PD) testing.
As a result, it showed weight-loss effects at a level similar to the retatrutide control group, and there was also a tendency for mitigated rapid weight regain after dosing stopped. The company said a once-monthly dosing cycle could be competitive in terms of patient adherence in a market dominated by once-weekly therapies.
— Jeon Tae-yeon, CEO of Alteogen