Axsome Begins Phase III AXS-14 FORWARD Trial for Fibromyalgia Management
Key Highlights
- Milestone: First patient dosed in the Phase III FORWARD trial.
- Candidate: AXS-14 (esreboxetine), a selective norepinephrine reuptake inhibitor.
- Methodology: Randomised withdrawal design to assess time to loss of response.
- Patient Base: Targets 17 million people in the US with limited treatment options.
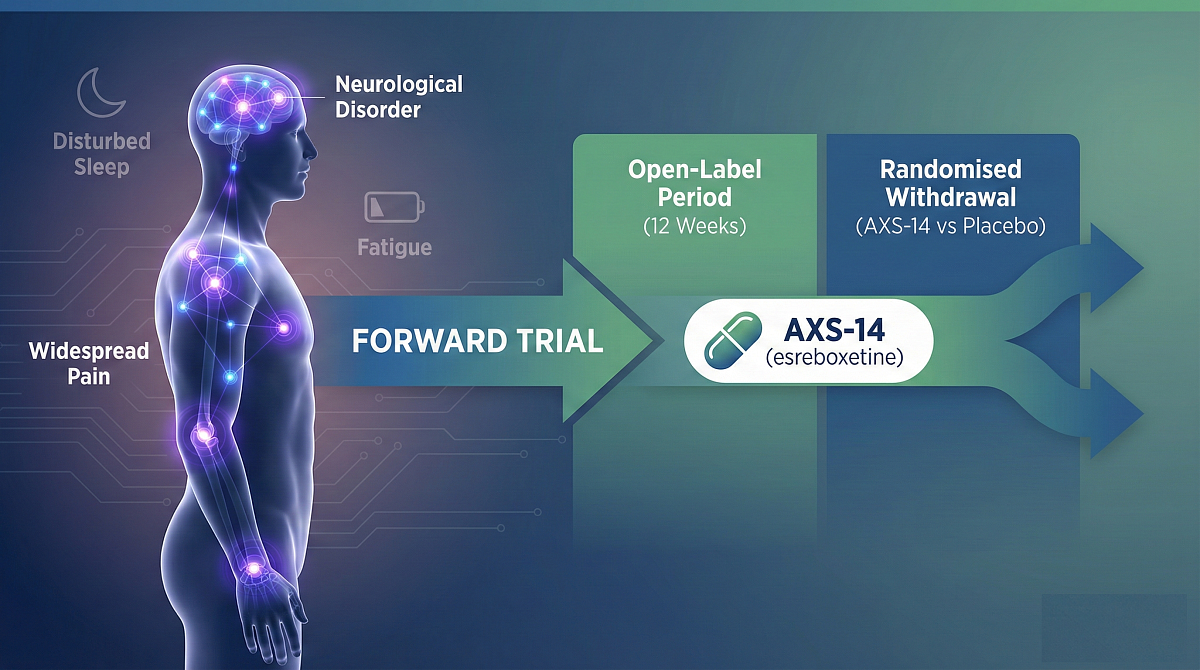
Axsome Therapeutics has initiated the FORWARD Phase III trial evaluating AXS-14 (esreboxetine, synonym PNU-165442G) for the management of fibromyalgia. The company announced that the first patient is now dosed, launching a pivotal study for this widespread neurological pain disorder.
Study Design & Methodology
The FORWARD Phase III trial employs a randomised withdrawal research design. This multi-centre, double-blind, placebo-controlled study assesses efficacy through a structured two-stage process:
Fibromyalgia: The Unmet Need
Fibromyalgia affects approximately 17 million individuals in the US. It is characterised by widespread pain, disturbed sleep, and hypersensitivity. The condition significantly impacts physical, emotional, and social well-being, leading to economic challenges and a lower quality of life.
Current treatment choices are limited. More than half of patients stop therapy within one year due to insufficient symptom relief or unacceptable side effects. Common symptoms include:
About AXS-14 (Esreboxetine)
AXS-14 is a selective norepinephrine reuptake inhibitor (NRI). The active agent, esreboxetine, is the SS-enantiomer of racemic reboxetine.
Note: AXS-14 is an investigational product and has not yet been approved by the FDA.