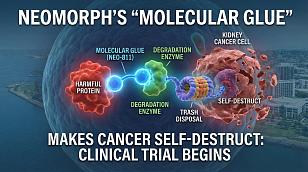
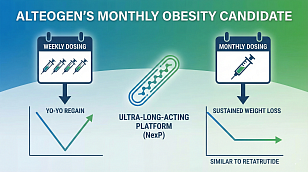
Clinical Trials: The Indispensable Cornerstone of Modern Medical Progress
In 1747, Dr. James Lind established the foundation of evidence-based research. By proving that citrus fruits cured scurvy aboard a British naval vessel, he created the methodology that remains the backbone of the pharmaceutical industry today.
Clinical trials are much more than a procedural step; they are the core of the research-based pharmaceutical industry. Every scientific breakthrough must be validated through rigorous human studies to ensure that innovation is rooted in clinical evidence before reaching patients worldwide.
Responsible clinical trial data sharing is a vital enabler of progress. By reducing redundant research and safeguarding patient privacy, the industry fosters an environment of absolute transparency. This commitment rests on three pillars:
- ✔ Safeguarding patient confidentiality and data privacy.
- ✔ Upholding the scientific integrity of global regulators.
- ✔ Sustaining investment in high-risk biomedical innovation.
Modern research demands that trial populations reflect the epidemiology and demographics of those who stand to benefit. At ChemDiv and across the global biopharma sector, advancing diversity is seen not only as a matter of equity but as essential scientific rigor—ensuring the right treatment for the right patient.