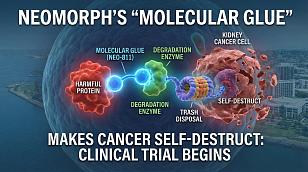
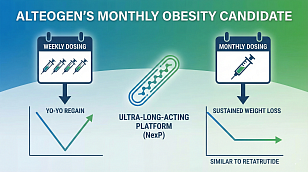
FDA Grants Breakthrough Therapy Status to New Gene Therapy for Dravet Syndrome
The U.S. Food and Drug Administration (FDA) has officially granted Breakthrough Therapy Designation to ETX101, an experimental, one-time gene therapy developed by Encoded Therapeutics. This milestone underscores the urgent need for disease-modifying treatments for Dravet syndrome, a rare and devastating form of epilepsy.
Dravet syndrome is primarily caused by mutations in the SCN1A gene, leading to disrupted nerve signaling and severe seizures. ETX101 is designed to address the underlying cause by delivering a healthy copy of the SCN1A gene directly to nerve cells via a one-time infusion into the brain's fluid-filled spaces.
Encoded Therapeutics is currently evaluating the safety and efficacy of ETX101 through the comprehensive POLARIS program. This clinical framework consists of three distinct studies targeting children with Dravet syndrome.
Interim results from 19 children treated across the Phase 1/2 studies have been promising. Durable reductions in seizure frequency were observed alongside improvements in communication abilities and cognitive measures. Crucially, no serious side effects have been reported to date.
Beyond the Breakthrough Therapy status, the FDA has recognized the potential of ETX101 through multiple advanced designations designed to accelerate market access:
The Breakthrough status allows for more frequent interaction and guidance from the FDA. Encoded Therapeutics continues to collaborate closely with regulators to bring this disease-modifying option to the Dravet syndrome community as rapidly as possible.