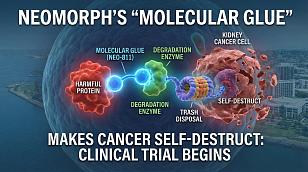
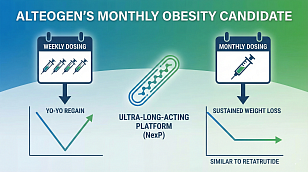
FDA Issues Landmark Guidance to Modernize Clinical Trials Through Bayesian Methodologies
In a significant step toward transforming drug development, the U.S. Food and Drug Administration (FDA) has published draft guidance aimed at facilitating the use of Bayesian methodologies in clinical trials for drugs and biologics. This initiative is designed to help developers leverage existing data more effectively, optimize trial efficiency, and accelerate the delivery of safe treatments to patients.
Bayesian approaches provide a sophisticated framework by combining study data with relevant prior information to create a new distribution for inference. This modern statistical shift is key to addressing the dual challenges of high research costs and prolonged clinical timelines.
The guidance outlines several critical ways Bayesian methods can be integrated into innovative trial designs to support primary inference and decision-making:
Bayesian methods are particularly valuable for sponsors targeting rare diseases or pediatric indications. In these scenarios, where patient populations are inherently small, the ability to incorporate external data sources can provide the statistical power necessary to draw robust conclusions about safety and efficacy.
The release of this guidance — "Use of Bayesian Methodology in Clinical Trials of Drugs and Biologics" — satisfies a core commitment under the Prescription Drug User Fee Act (PDUFA) VII. As part of this reauthorization, the FDA pledged to enhance its capacity to review complex innovative trial designs.
The FDA is currently seeking public comment on this draft guidance. Stakeholders are encouraged to provide feedback to ensure these modern statistical methods translate into tangible public health impacts and streamlined regulatory pathways.