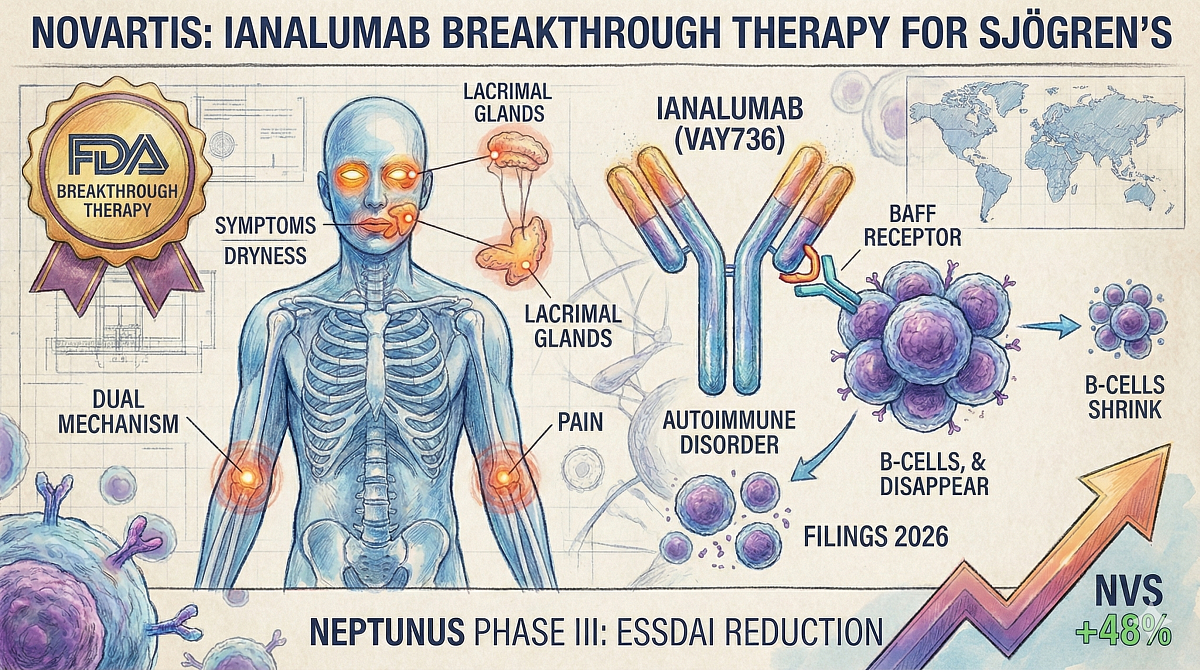
Novartis' Ianalumab Wins Breakthrough Therapy Tag for Sjögren's Disease
- Type: Monoclonal Antibody (mAb)
- Mechanism: Dual-action (BAFF receptor targeting & B-cell depletion)
- Status: FDA Breakthrough Therapy
- Trial Data: Met primary endpoints in NEPTUNUS-1 & 2
- Filings: Expected early 2026
Clinical Efficacy: The NEPTUNUS Studies
The Breakthrough Therapy designation is supported by robust data from the Phase III NEPTUNUS-1 and NEPTUNUS-2 studies. These global, multicenter, pivotal trials met their primary endpoints, demonstrating clinically meaningful and statistically significant improvements in disease activity.
Efficacy was measured by a reduction in the ESSDAI score (EULAR Sjögren's Syndrome Disease Activity Index), a widely accepted clinical tool used to measure systemic disease activity. The results showed significant ESSDAI reductions in patients treated with ianalumab compared with placebo.
Mechanism of Action: Dual B-Cell Targeting
Ianalumab is an investigational monoclonal antibody with a novel dual mechanism of action. It is engineered to:
- Deplete B-cells by directly targeting the BAFF receptor.
- Inhibit B-cell activation and survival, interrupting the autoimmune cycle.
Regulatory & Commercial Pathway
Novartis intends to submit regulatory applications to global health authorities, including the FDA, to commercialize ianalumab beginning in early 2026. If approved, it would address a significant unmet need, as there are currently no approved disease-modifying therapies for Sjögren’s disease.
The asset was added to Novartis’ pipeline following a collaboration with MorphoSys AG, which was fully acquired by the company in 2024. The FDA had previously granted Fast Track designation to ianalumab in 2016.
Market Impact & Pipeline Expansion
Beyond Sjögren’s disease, ianalumab is being evaluated for multiple B-cell-driven autoimmune diseases. The candidate has shown promising efficacy and a favorable safety profile in studies for:
- Systemic Lupus Erythematosus (SLE)
- Immune Thrombocytopenia (ITP)
- Lupus Nephritis
- Warm Autoimmune Hemolytic Anemia
About Sjögren’s Disease
Sjögren’s disease is a progressive, heterogeneous, rheumatic autoimmune disorder. It causes inflammation and tissue damage across multiple organs, characterized by symptoms such as severe dryness, fatigue, and pain. The condition significantly reduces quality of life and carries an increased risk of lymphoma.