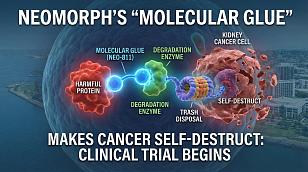
FDA Issues Urgent Nationwide Recall of Modern Warrior Energy Supplements Due to Illegal Drug Contamination
The U.S. Food and Drug Administration (FDA) has announced a critical recall of "Ready" supplements manufactured by Arizona-based company Modern Warrior. Marketed as tools for enhancing mental clarity and metabolic energy, these products were found to contain undeclared synthetic drugs that pose significant life-threatening risks to consumers.
The recall follows laboratory testing that identified three potent, unapproved pharmacological agents within the supplement formulation: Tianeptine, 1,4-DMAA, and Aniracetam. These substances are not permitted in dietary supplements and can cause severe cardiovascular and neurological events.
Unlike prescription medications, dietary supplements sold by companies like Modern Warrior do not undergo FDA pre-market approval. This critical gap allows products to reach consumers without established efficacy or safety data from large-scale clinical trials.
| Requirement | Dietary Supplements | Prescription Drugs |
|---|---|---|
| Pre-market Clinical Trials | Not Required | Mandatory |
| Efficacy Demonstration | Manufacturer Claim Only | FDA Verified |
| Ingredient Transparency | Often Undeclared | Strictly Monitored |
According to federal records, the affected Modern Warrior "Ready" supplements—which retail for approximately $170 per set—were distributed nationwide between April 2022 and December 2025. The FDA warns that hundreds of thousands of consumers may be affected by this contamination.
Modern Warrior, based in Scottsdale, Arizona, has reportedly ceased all sales and moved remaining inventory to a "quarantine" area of its warehouse. All online listings for the supplement set have been disabled to prevent further accidental distribution.
Consumers in possession of these supplements are urged to stop use immediately and contact the manufacturer for disposal instructions. Any adverse health events should be reported to the FDA’s MedWatch program to assist in the ongoing investigation.