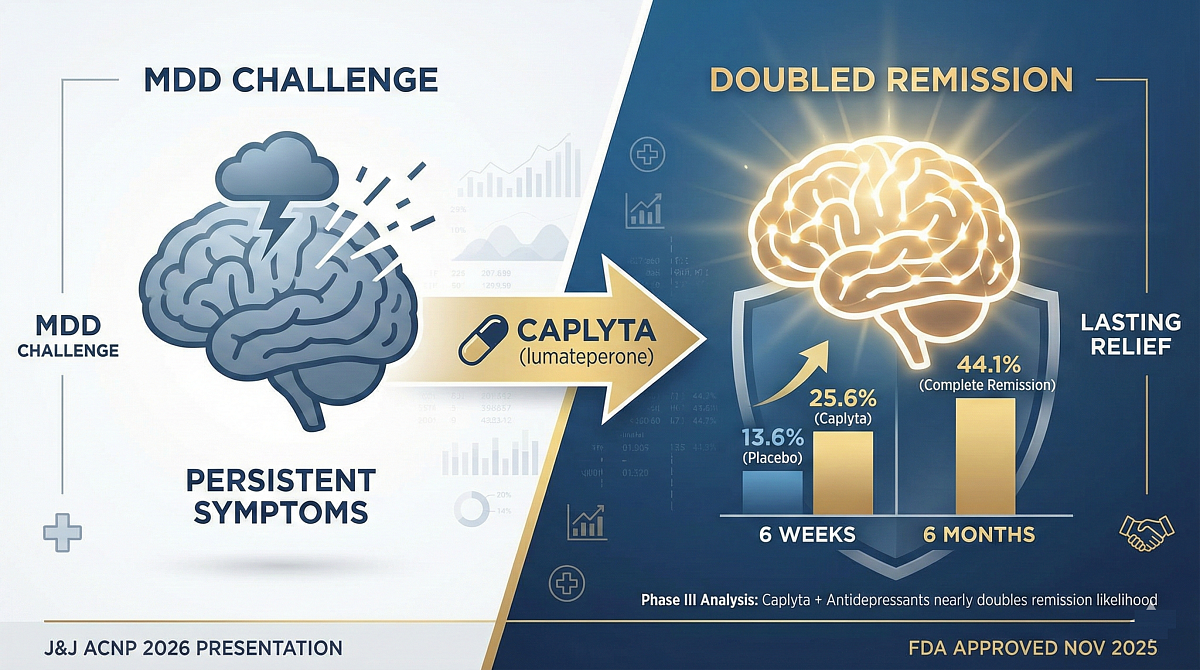
NASSAU, Bahamas — Johnson & Johnson (J&J) presented transformative data from three Phase III trials at the 64th Annual Meeting of the ACNP. The analysis reveals that Caplyta (lumateperone), used as an adjunctive therapy, nearly doubled the likelihood of remission in patients suffering from MDD.
The pooled data from trials NCT04985942, NCT05061706, and NCT05061719 showed that 10.6% of patients achieved complete remission in the Caplyta cohort, compared to just 5.6% in the placebo group. This benefit was observed after only six weeks of treatment.
Durability of Response (6-Month Extension)
Efficacy was not only significant but maintained over time. In a six-month safety study extension:
- 66% (Nearly 2 out of 3) of patients reached MADRS ≤10.
- 44.1% achieved complete remission.
- 42.8% of patients experienced sustained remission by the end of treatment.
J&J acquired Caplyta following its $14.6bn takeover of Intra-Cellular Therapies in April 2025. This acquisition, the largest pharma M&A transaction of the year, underscores J&J's commitment to dominating the neuropsychiatry market. The FDA granted MDD adjunctive therapy approval in November 2025.
2029 Market Projections
The MDD market is expected to reach $9.55bn by 2029 (7.3% CAGR).
Est. Caplyta Sales