Novel Small Molecule Inhibitors Demonstrate Compelling Efficacy in Endometrial Cancer
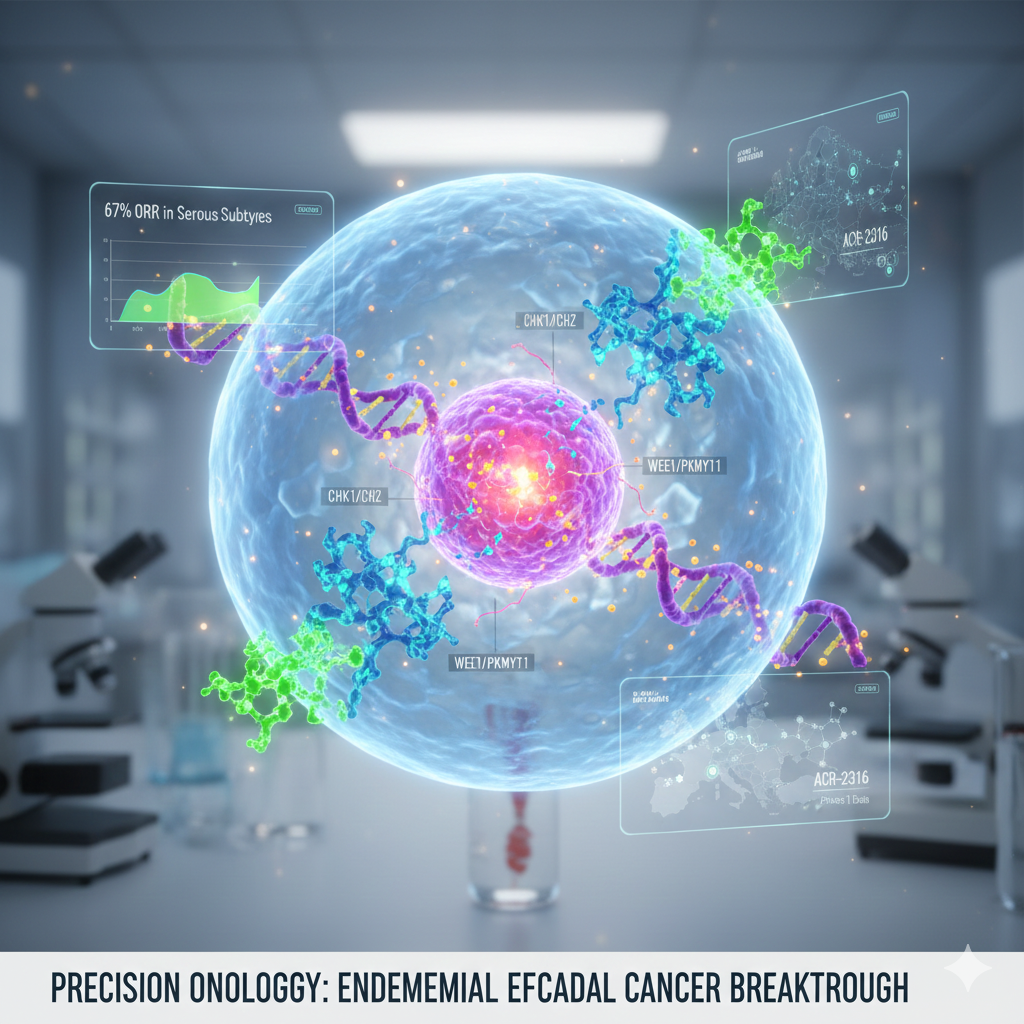
Recent data from clinical evaluations of two innovative oncology assets, Prexasertib (ACR-368) and ACR-2316, have signaled a major advancement in the treatment of Endometrial Cancer (EC). Specifically, targeted inhibition of cell cycle checkpoints is emerging as a powerful strategy for addressing challenging serous subtypes that historically lack effective therapeutic options.
Prexasertib (ACR-368): Targeting CHK1/2 via Precision Proteomics
Prexasertib is a selective small molecule inhibitor targeting CHK1 and CHK2. Its development is uniquely driven by Acrivon Therapeutics’ AP3 (Acrivon Predictive Precision Proteomics) platform, which identifies patients most likely to respond based on specific protein signatures.
Biological insights suggest that serous tumors are particularly sensitive to CHK1/2 inhibition due to their G1-S checkpoint deficiency, making them heavily dependent on the G2-M checkpoint for DNA repair. By disrupting this dependency, Prexasertib induces mitotic catastrophe in malignant cells while minimizing impact on healthy tissue.
39% ORR in all-comer EC populations.
52% confirmed ORR in all-comer serous subtypes.
Clinical Trial Applications approved for Germany, Italy, France, and Spain (20+ sites).
Strategic Evolution: Arm 3 Clinical Trial Design
Based on heightened sensitivity in serous tumors, the trial is expanding its third arm to exclusively enroll subjects with serous EC. This arm will evaluate Prexasertib in combination with ultra-low dose gemcitabine, acting as a tumor sensitizer. Key features include:
- No Pretreatment Biopsy: Streamlined enrollment to accelerate recruitment.
- Optimized Timeline: Patient enrollment in the EU is expected in Q1 2026, with completion projected by Q4 2026.
- Fast Track Status: The FDA has granted Fast Track designation to Prexasertib for platinum-resistant ovarian and endometrial cancers.
ACR-2316: A Potential First-in-Class WEE1/PKMYT1 Inhibitor
The company’s second asset, ACR-2316, has completed a Phase 1 study (NCT06667141) showing a favorable tolerability profile. As a dual WEE1/PKMYT1 inhibitor, it addresses multiple nodes in the DNA damage response (DDR) pathway. Initial signs of efficacy included Partial Responses (PR) in tumor types previously resistant to this class of inhibitors.
Impact on Drug Discovery and R&D
For organizations like ChemDiv, the success of these small molecule inhibitors validates the "Precision Proteomics" approach in drug discovery. By moving beyond genomic sequencing to functional protein analysis, developers can identify niche patient populations where efficacy is exponentially higher, such as the serous subtype in EC.
As these trials progress through 2026, the integration of selective CHK1/2 and WEE1 inhibitors into standard care could redefine the oncology landscape for patients with high-burden gynecological malignancies.
Ongoing EU expansions and FDA Fast Track support reflect the high unmet medical need and the clinical significance of these novel therapeutic assets.