First Preclinical Development Candidate for Nucleome Therapeutics: First-in-class Monoclonal Antibody Agonist for Inflammation
The nomination follows a period of intense scientific progress for the company, which utilizes cutting-edge 3D human genetics to uncover the molecular mechanisms driving disease.
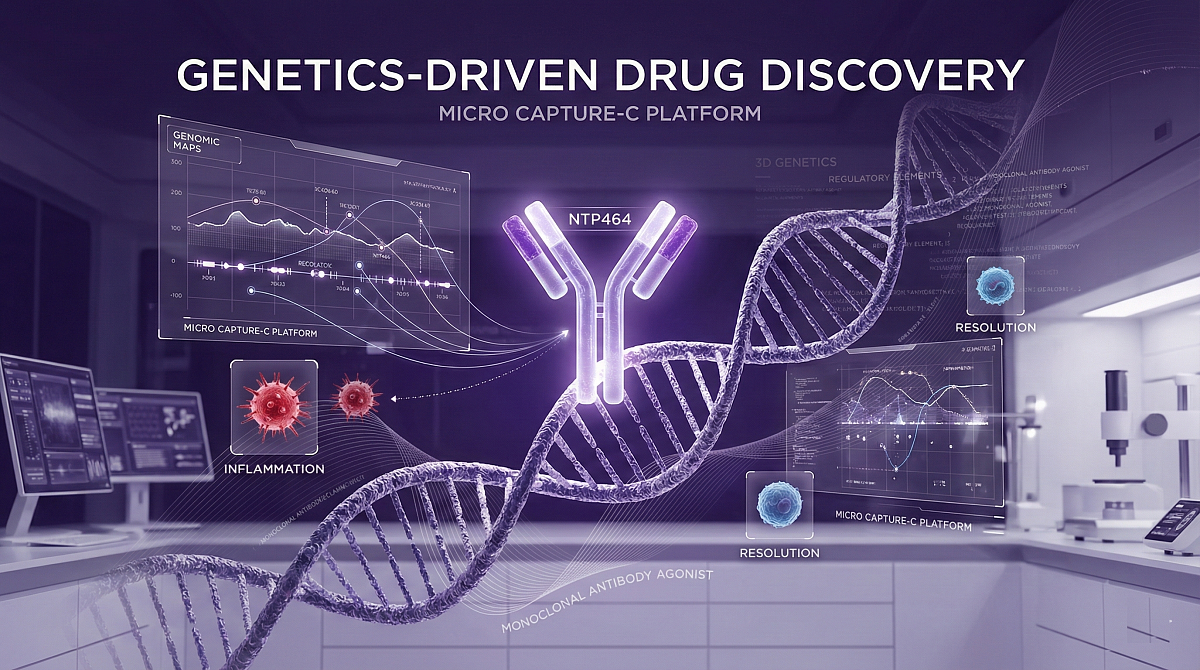
Central to Nucleome’s success is its proprietary Micro Capture-C (MCC) platform. This lab-based technology maps physical interactions within the genome at unprecedented resolution. When combined with machine learning and advanced computational analysis, MCC enables scientists to decipher exactly how non-coding genetic variation influences gene regulation in human disease.
Uncovering a New Inflammation Checkpoint
NTP464 targets a previously unrecognized inflammation checkpoint identified using Nucleome’s genetic methods. By analyzing how genetic variants associated with autoimmune disease alter three-dimensional interactions between regulatory elements and protein-coding genes, the company uncovered a key multi-cellular regulator.
In healthy individuals, this mechanism restores immune balance. However, it becomes dysregulated in chronic diseases such as:
- Rheumatoid Arthritis
- Systemic Lupus Erythematosus (SLE)
- Inflammatory Bowel Disease (IBD)
Preclinical Efficacy: Resolving, Not Just Suppressing
The nominated monoclonal antibody agonist has demonstrated strong potency, selectivity, and developability. Preclinical testing revealed a unique dual-action profile:
These effects support a novel therapeutic principle focused on actively resolving inflammation rather than simply suppressing immune responses. The NTP464 program will now progress towards investigational new drug (IND)-enabling studies.