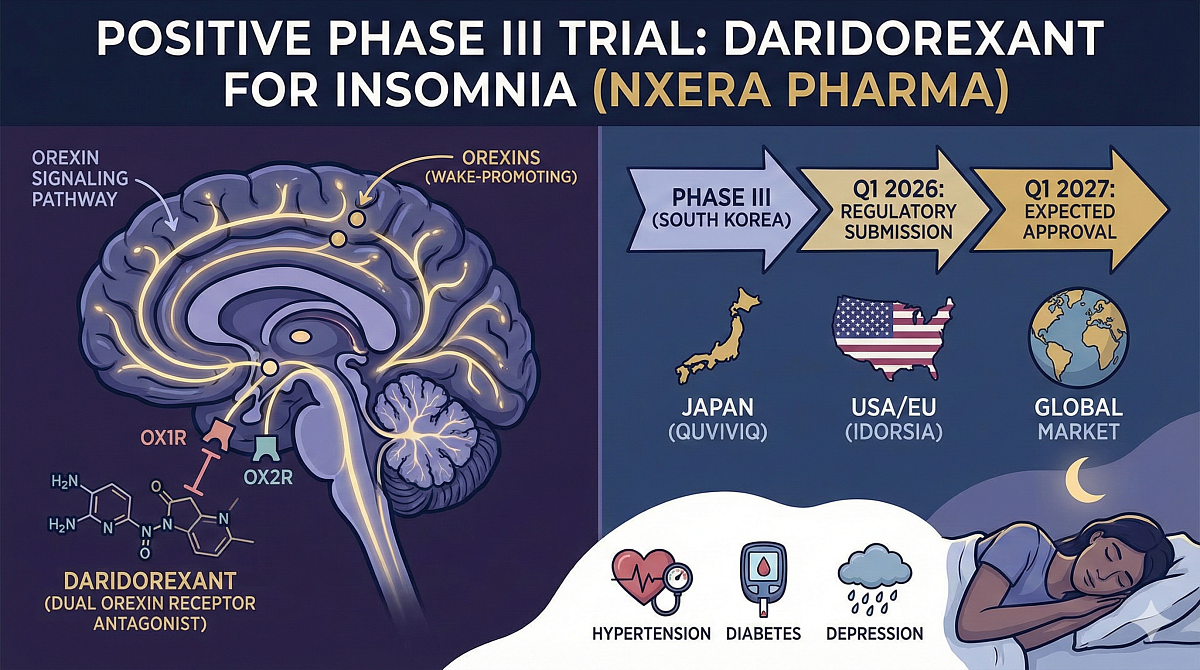
Positive Phase III Trial of Nxera’s Daridorexant: New Hope for Insomnia Patients in South Korea
Japan-based Nxera Pharma has announced that positive top-line results were obtained from a pivotal Phase III study conducted in South Korea. The trial evaluated the efficacy and safety of daridorexant (50mg) in adult and elderly patients suffering from insomnia.
Based on these promising results, Nxera is moving forward with regulatory submissions. The company has outlined a clear path to commercialization in the region:
Scientific Deep Dive: Mechanism of Action
Daridorexant (formerly known as nemorexant) represents a class of novel drugs that target the orexin signaling pathway. Unlike conventional treatments, it functions as a selective dual orexin receptor antagonist.
It works on receptors OX1R and OX2R to block the binding of orexins—endogenous neuropeptides that promote wakefulness. This mechanism was refined through an intensive drug discovery program to maximize the duration of action while minimizing the "hangover" effect.
| Treatment Class | Target | Common Side Effects |
|---|---|---|
| Conventional Hypnotics | GABA-A, Serotonin, Histamine | Residual sleepiness, motor incoordination, falls, memory/cognitive impairment. |
| Daridorexant (Nxera) | Orexin Receptors (Dual Antagonist) | Designed to improve potency while minimizing next-morning residual activity. |
Addressing the Burden of Insomnia
Insomnia is characterized not just by difficulties with sleep onset or maintenance, but by significant impairment of daytime functioning. It chronically affects daily life and is associated with serious long-term comorbidities, including:
- Hypertension
- Diabetes
- Depression
Novel drugs targeting orexin receptors gained attention after the discovery of the pathway's role in wakefulness and early compounds like [almorexant]. With the positive Phase III results in South Korea, Nxera Pharma aims to bring this targeted therapy to a broader patient population.