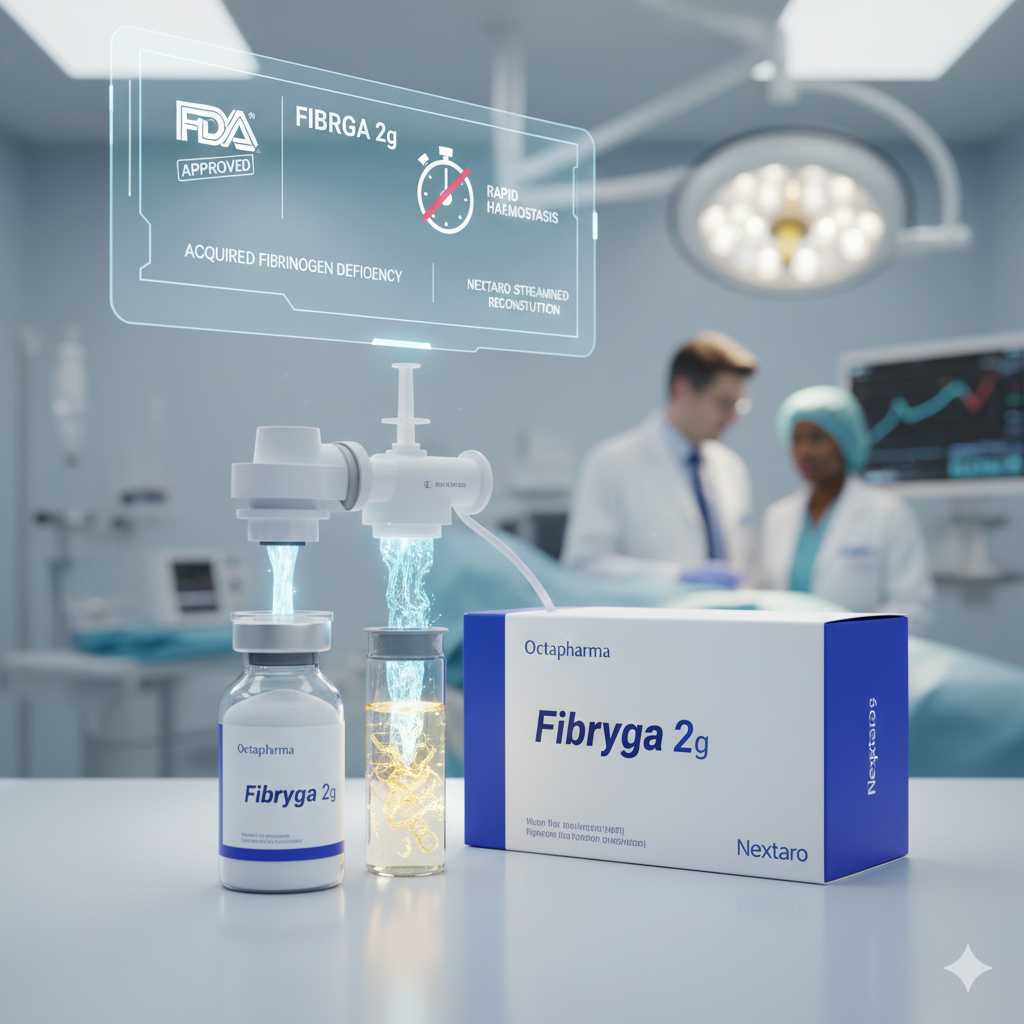
Octapharma Receives FDA Approval for New 2-Gram Fibryga Formulation in AFD
In a significant advancement for emergency haematology, Octapharma has received approval from the US Food and Drug Administration (FDA) for a new 2-gram (g) formulation of Fibryga. This human plasma-derived fibrinogen concentrate is specifically authorised for the treatment of patients with Acquired Fibrinogen Deficiency (AFD), providing a critical tool for managing acute bleeding complications.
Streamlining Urgent Care: The New 2g Reconstitution Kit
The introduction of the 2g kit is designed to enhance dosing flexibility and speed in high-pressure clinical environments. Key technical features of the new approval include:
- Nextaro Reconstitution Device: Included in the kit to enable streamlined preparation and precise delivery.
- 100ml Water for Infusion (WFI): Optimized for quick dissolution of the lyophilised powder.
- Reduced Preparation Time: Allows healthcare providers to act faster in "time-critical" bleeding scenarios.
Authorised for both Congenital and Acquired Fibrinogen Deficiency.
Supported by clinical data published in JAMA, validating efficacy in AFD.
A New Standard in Haemostasis Management
AFD is a life-threatening condition where fibrinogen levels drop rapidly during major surgery or trauma, leading to uncontrolled haemorrhage. Traditionally, cryoprecipitate was the primary treatment, but it often lacks viral inactivation and requires time-consuming thawing. Fibryga addresses these limitations through:
- Viral Safety: Sole virus-inactivated concentrate on the US market for this indication.
- Rapid Deployment: Lyophilised form allows for storage at point-of-care and immediate reconstitution.
- Precision Dosing: The new 2g option complements the existing 1g version, allowing for more precise fibrinogen supplementation.
Strategic R&D Perspective
For drug discovery and clinical research organizations like ChemDiv, Octapharma's investment in advanced haemostasis products underscores the move toward safer, faster-acting biologics. Enhancing the delivery mechanisms of plasma-derived therapies is a cornerstone of modern patient outcomes in acute care.
As Flemming Nielsen, President of Octapharma, stated: "By simplifying preparation and expanding dosing flexibility, we’re helping providers act faster and more precisely – when every second counts."
Fibryga is indicated for the treatment of acute bleeding episodes in patients with congenital afibrinogenaemia and hypofibrinogenaemia, as well as acquired deficiency.