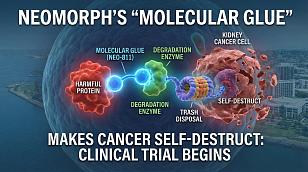
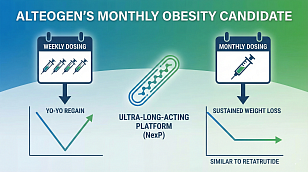
Phase 3 Clinical Trial Reporting & Prospective Regulatory Filings to Watch For in 2026
The year 2026 promises to be a pivotal period for pharmaceutical innovation, with numerous clinical trial data readouts, study initiations, and anticipated FDA approvals for groundbreaking therapeutics and vaccines. Below is a curated overview of key candidates either poised to enter Phase 3 trials, report crucial data from ongoing Phase 3 studies, or seek regulatory approval from health agencies globally.
This year will highlight the acceleration of drug development, driven by advanced trial designs and pressing public health needs. From infectious diseases to chronic conditions, the pipeline reflects significant progress towards new treatment paradigms.
VLA15 (Pfizer & Valneva)
The VLA15 vaccine candidate is currently in a Phase 3 trial (VALOR) that has completed its primary observation period for disease occurrence. Conducted across highly endemic regions in the US, Canada, and Europe, this multicenter, placebo-controlled study evaluates efficacy, safety, and immunogenicity. Data expected to be reported this year.
Status: Phase 3 Data Readout Anticipated
Bemnifosbuvir + Ruzasvir (Atea Pharma)
Atea Pharmaceuticals recently reported compelling modeling data for its investigational combination of bemnifosbuvir (BEM) and ruzasvir (RZR), projecting near-complete suppression of HCV activity within 7 to 8 weeks. The first Phase 3 topline results for this potent antiviral combination are expected mid-2026.
Status: Phase 3 Topline Results Expected
Zosurabalpin (Roche)
Roche has announced plans to initiate a Phase 3 trial for its novel antibiotic candidate, zosurabalpin, targeting Carbapenem-Resistant Acinetobacter baumannii (CRAB) infections. This represents a critical development in the fight against antimicrobial resistance, with the trial launch slated for early 2026.
Status: Phase 3 Trial Launch Expected
Bictegravir + Lenacapavir (Gilead Sciences)
Gilead Sciences released positive topline results from its Phase 3 ARTISTRY-2 trial, evaluating a fixed-dose combination of bictegravir (BIC) and lenacapavir (LEN) for virologically suppressed adults with HIV. Following these robust results, Gilead plans to submit data for regulatory approval, marking a potential new option for HIV treatment.
Status: Regulatory Filing Anticipated
The outcomes of these trials and subsequent regulatory decisions will significantly shape the future treatment landscape across multiple disease areas, offering new hope to patients worldwide.