Vibrant Therapeutics Raises $61m in Funding to Advance Intelligent Therapeutics Pipeline
New Financing
$61 MillionTotal Capital Raised
$100 MillionKey Investors
• Apricot Capital
The financing round included participation from new high-profile investors Pfizer Ventures and Apricot Capital. The funds will be utilized to advance Vibrant's pipeline of intelligently designed therapeutics and further develop its proprietary platform for drug design.
FDA IND Acceptance for VIB305
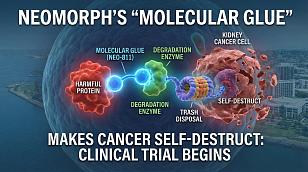
Alongside the funding news, Vibrant announced a significant regulatory milestone: the US Food and Drug Administration (FDA) has accepted its Investigational New Drug (IND) application for the company's lead programme, VIB305.
About VIB305 Phase 1 (AUS/CN) IND Accepted (USA)
VIB305 is a masked T-cell engager (TCE) prodrug designed with precision in mind. Its mechanism of action involves:
- Selective Activation: Activates specifically within the microenvironment of a tumour.
- Tissue Sparing: Designed to spare healthy tissues surrounding the tumour.
- Target Indication: Epidermal growth factor receptor (EGFR)-expressing solid tumours.
Current Status: VIB305 is already being studied in Phase 1 clinical trials in Australia and China. The FDA acceptance now clears the path for US clinical trials.