PDZ PPI Library
ChemDiv’s library of small molecule compounds targeting PDZ-domains for protein-protein interaction modulation comprises 3,055 entries.
PDZ domains are prevalent protein interaction modules capable of recognizing short amino acid motifs at the C-termini of target proteins, playing a key role in regulating numerous biological processes.
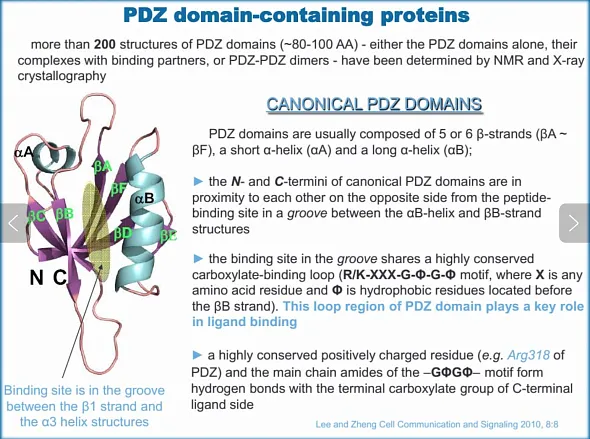
Typically, PDZ domains are comprised of 80-100 amino acid residues and share a common topology, consisting of six β-strands (βA to βF), a short α-helix (αA), and a longer α-helix (αB). The N- and C-termini of canonical PDZ domains are positioned in proximity to each other, on the opposite side of the peptide-binding groove, which is located between the αB-helix and the βB-strand. This domain harbors a single binding site located in the groove between the αB and βB structural elements, featuring a highly conserved carboxylate-binding loop preceding the βB strand. The loop region within the PDZ domain is crucial for ligand binding. Proteins containing PDZ domains can interact with a multitude of other proteins. The regulatory mechanisms influencing PDZ domain-mediated protein-protein interactions include phosphorylation, disulfide bond formation, autoinhibition, competitive binding, and allostery [1].
Protein-protein interactions (PPIs) are essential for cellular functionality, and their dysregulation has been implicated in numerous diseases. Given their well-defined binding sites, PDZ domains emerge as promising targets for therapeutic intervention. This domain is found 257 times across 142 human proteins. Several PDZ domains have been linked to the progression of diseases, including cancer, cystic fibrosis, schizophrenia, Alzheimer's, Parkinson's disease, avian influenza, pain, and stroke, often through interactions with disease-associated proteins. To address the limitations associated with peptides, the utilization of small molecules has been explored. The involvement of PDZ domains in disease pathways underscores the potential for developing inhibitors that selectively target PDZ-mediated interactions, thereby modulating PPIs. A notable example of such a small molecule is indole 8, which was identified through virtual library screening. It is capable of inhibiting the PDZ2 domain of MAGI3, an adaptor protein crucial for the regulation of tissue organization and differentiation, demonstrating the feasibility of this approach in drug discovery.
Our small molecule library targeting PDZ domains represents a powerful tool in drug discovery, offering several significant benefits. Firstly, they provide a versatile platform for identifying inhibitors that can modulate PPIs critical for numerous cellular processes and implicated in various diseases, including cancer, neurological disorders, and infectious diseases. By selectively targeting PDZ domains, our library enables the discovery of compounds with high specificity, potentially reducing off-target effects and associated toxicity. Furthermore, the small molecule approach overcomes the limitations associated with peptide-based therapies, such as poor cell permeability and stability, offering a more practical route to therapeutic intervention. Additionally, the development of drugs using our library facilitates the exploration of the structural and functional diversity of PDZ domains across different proteins, enhancing our understanding of these domains' role in disease mechanisms. Ultimately, small molecule libraries targeting PDZ domains hold the promise of yielding novel therapeutics with the potential to selectively interfere with disease-relevant PPIs, paving the way for targeted and effective treatments [2].
References
[1] H. J. Lee and J. J. Zheng, “PDZ domains and their binding partners: Structure, specificity, and modification,” Cell Communication and Signaling, vol. 8, no. 8. BioMed Central, pp. 1–18, May 28, 2010, doi: 10.1186/1478-811X-8-8.
[2] S. Ducki and E. Bennett, “Protein-Protein Interactions: Recent Progress in the Development of Selective PDZ Inhibitors,” Curr. Chem. Biol., vol. 3, no. 2, pp. 146–158, 2009, doi: 10.2174/2212796810903020146.