Aldeyra Therapeutics Receives Orphan Drug Designation from the U.S. Food and Drug Administration for ADX-2191 to Treat Retinitis Pigmentosa
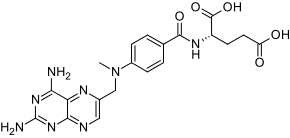
LEXINGTON, Mass.--(BUSINESS WIRE)-- Aldeyra Therapeutics (Nasdaq: ALDX) (Aldeyra) today announced that the U.S. Food and Drug Administration (FDA) has granted orphan drug designation for ADX-2191 (methotrexate for intravitreal injection) for the treatment of retinitis pigmentosa (RP). There are no approved drug treatments for patients with RP, a clinical group of rare genetic eye diseases characterized by retinal cell death and loss of vision. The disease affects an estimated 82,000-110,000 individuals in the United States, and approximately 1 in 4,000 people worldwide.
“Retinitis pigmentosa is a serious and incurable sight-threatening disease that represents a major unmet need in the field of ophthalmology,” stated Todd C. Brady, M.D., Ph.D., President and CEO of Aldeyra. "ADX-2191 has now received orphan designations for three distinct clinical indications, highlighting the broad platform potential of ADX-2191 to treat an array of rare retinal disorders.”
Methotrexate inhibits dihydrofolic reductase, an enzyme involved in cellular replication and activation. In addition to RP, the FDA has granted orphan drug designation to ADX-2191 for the treatment of primary vitreoretinal lymphoma, a rare, aggressive, high-grade cancer; and both orphan drug and fast track designation to ADX-2191 for the prevention of proliferative vitreoretinopathy, a rare inflammatory disorder of the retina that leads to severe retinal scarring and blindness and is the leading cause of failure of retinal reattachment surgery.
The FDA’s orphan drug designation program is designed to provide financial incentives to sponsors for developing drugs and biologics for rare diseases and conditions, in part defined as affecting fewer than 200,000 people in the United States. Sponsors of designated orphan drugs are eligible for partial tax credits for clinical trial costs, waiver of the user fee for marketing applications and, upon approval, consideration for seven years of marketing exclusivity.
About Aldeyra Therapeutics
Aldeyra Therapeutics is a biotechnology company developing novel immune-modulating therapies to treat ocular and systemic diseases. Two of the company’s lead product candidates, reproxalap and ADX-629, target RASP (reactive aldehyde species), which are pre-cytokine, systems-based mediators of inflammation. Reproxalap is being evaluated in Phase 3 clinical trials in patients with dry eye disease and allergic conjunctivitis. The company’s clinical pipeline also includes ADX-2191 (methotrexate for intravitreal injection), a drug candidate in Phase 3 testing for the prevention of proliferative vitreoretinopathy.
Aug. 4, 2021
https://www.biospace.com/



