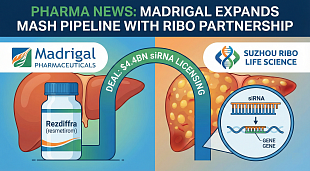
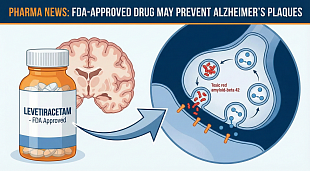
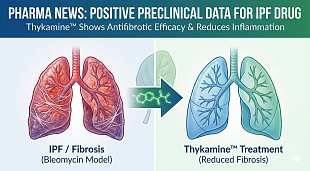
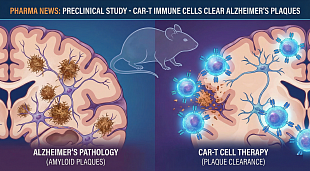
Bristol Myers Squibb Application for Zeposia® (ozanimod) for the Treatment of Ulcerative Colitis Accepted for Filing with Priority Review by U.S. Food and Drug Administration
PRINCETON, NJ, USA I February 01, 2021Bristol Myers Squibb (NYSE:BMY) today announced that the U.S. Food and Drug Administration (FDA) has accepted its supplemental New Drug Application (sNDA) for Zeposia® (ozanimod) for the treatment of adults with moderately to severely active ulcerative colitis (UC). Following the redemption of a Priority Review Voucher with the submission, the FDA assigned a Prescription Drug User Fee Act (PDUFA) goal date of May 30, 2021.
“People living with ulcerative colitis often struggle to manage their disease and can face a severe impact to their lives, highlighting a need for new oral treatment options with proven safety and efficacy profiles that can be utilized early in the treatment journey,” said Mary Beth Harler, M.D., head of Immunology and Fibrosis Development, Bristol Myers Squibb. “We look forward to continuing to work with the FDA to bring Zeposia to those living with this serious, chronic disease.”
The sNDA submitted to the FDA is based on results from True North, a pivotal, placebo-controlled Phase 3 trial evaluating Zeposia as an induction and maintenance therapy in adults with moderately to severely active UC. True North met both primary endpoints, demonstrating highly statistically significant and clinically meaningful results for clinical remission compared to placebo at induction at Week 10 and in maintenance at Week 52. The overall safety observed in True North was consistent with the known safety profile for Zeposia in approved labeling.
Bristol Myers Squibb thanks the patients and investigators who participated in the Zeposia Phase 3 UC True North clinical trial.
About Zeposia® (ozanimod)
Zeposia® (ozanimod) is an oral, sphingosine-1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. Zeposia reduces the capacity of lymphocytes to exit from lymph nodes, reducing the number of circulating lymphocytes in peripheral blood. The mechanism by which Zeposia exerts therapeutic effects in ulcerative colitis is unknown but may involve the reduction of lymphocyte migration into the inflamed intestinal mucosa.
Bristol Myers Squibb is continuing to evaluate Zeposia in an open-label extension trial, which is ongoing and designed to assess the longer-term profile of Zeposia for the treatment of moderately to severely active ulcerative colitis. The company is also investigating Zeposia for the treatment of moderately to severely active Crohn’s disease in the ongoing Phase 3 YELLOWSTONE clinical trial program.
Zeposia was approved by the U.S. Food and Drug Administration (FDA) for the treatment of adults with relapsing forms of multiple sclerosis (RMS) in March 2020. The European Commission approved Zeposia for the treatment of adult patients with relapsing remitting multiple sclerosis (RRMS) with active disease as defined by clinical or imaging features in May 2020. The European Medicines Agency validated Bristol Myers Squibb’s Marketing Authorization Application for Zeposia for the treatment of adults with moderately to severely active ulcerative colitis in December 2020. Zeposia is not approved for the treatment of ulcerative colitis in any country.
About Bristol Myers Squibb
Bristol Myers Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. For more information about Bristol Myers Squibb, visit us at BMS.com or follow us on LinkedIn, Twitter, YouTube, Facebook and Instagram.
Celgene and Juno Therapeutics are wholly owned subsidiaries of Bristol-Myers Squibb Company. In certain countries outside the U.S., due to local laws, Celgene and Juno Therapeutics are referred to as, Celgene, a Bristol Myers Squibb company and Juno Therapeutics, a Bristol Myers Squibb company.
SOURCE: Bristol-Myers Squibb
01 February 2021
https://pipelinereview.com/