FDA-approved gout medicine could also battle COVID-19
Probenecid is already FDA approved and has potent antiviral properties against SARS-CoV-2
As COVID-19 cases continue to skyrocket across the U.S. and the world, few options are available for treating patients infected with the SARS-CoV-2.
But new research from the University of Georgia offers hope for a viable therapeutic to combat the disease that has claimed more than 4 million lives worldwide.
Published in Nature’s Scientific Reports, the study found that probenecid has broad antiviral properties, making it a prime candidate to combat not only SARS-CoV-2 infection but also other common and deadly respiratory viruses like RSV and flu.
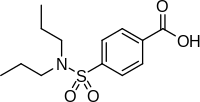
Probenecid is an FDA-approved medication that’s primarily used to treat gout, and it’s already widely available in the U.S. The drug has been on the market for over 40 years and has minimal side effects.
“There’s really nothing out there to safely fight these viruses,” said Ralph Tripp, lead author of the study and GRA Eminent Scholar of Vaccine and Therapeutic Studies in UGA’s College of Veterinary Medicine. “This antiviral works for all RNA respiratory viruses we tested, including SARS-CoV-2. RSV, coronavirus and flu all circulate in the same season. Bottom line is you can potentially reduce infection and disease using this one oral drug.”
11 Sept 2021
https://news.uga.edu/



