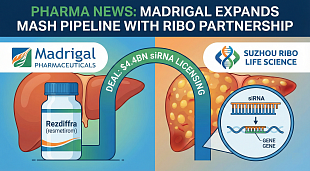
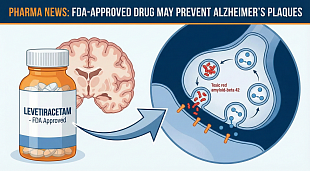
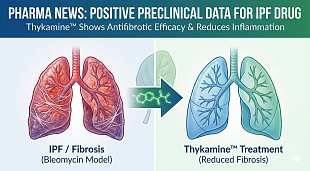
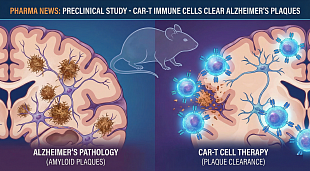
FDA Lifts Trial Hold on Aprea’s NonHodgkin’s Lymphoma Drug
Aprea Therapeutics has received FDA clearance to continue its eprenetapopt development, with the lifting of a full clinical hold placed last summer on the drug’s nonHodgkin’s lymphoma clinical trial program.
The company didn’t specify why the hold was instituted but said it has addressed the FDA’s concerns. It’s investigating eprenetapopt (APR-246) as add-on therapy in conjunction with azacitidine for myeloid malignancies.
Eprenetapopt, the company’s lead candidate, aims to reactivate the body’s natural tumor suppressor protein on gene p53.
Tumor protein gene p53 (TP53) plays a crucial role in the suppression of cancer growth. The gene also can determine the type of myeloid malignancy a person has, their treatment response and risk of the cancer transforming to acute myeloid leukemia (AML).
December 13, 2021