Inventiva announces FDA decision that Fast Track designation granted to lanifibranor in NASH encompasses the treatment of NASH with compensated cirrhosis
Daix (France), Long Island City (New York, United States), September 21, 2021 – Inventiva (Euronext Paris and Nasdaq: IVA), a clinical-stage biopharmaceutical company focused on the development of oral small molecule therapies for the treatment of non-alcoholic steatohepatitis (NASH), mucopolysaccharidoses (MPS) and other diseases with significant unmet medical need, today announced that the U.S. Food and Drug Administration (FDA) has decided that the Fast Track designation previously granted to lanifibranor in NASH encompasses the treatment of NASH patients with compensated cirrhosis.
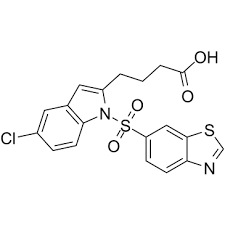
This decision follows a Fast Track designation request for lanifibranor in NASH with compensated cirrhosis filed by Inventiva with the FDA in August 2021. Previously, the FDA had granted both Fast Track and Breakthrough Therapy designations to lanifibranor for the treatment of NASH in September 2019 and October 2020, respectively.
The FDA’s dedicated Fast Track program is designed to facilitate the development and expedite the review and potential approval of drug candidates demonstrating the capacity to treat serious conditions and fill significant unmet medical needs.
Pierre Broqua, Ph.D., Chief Scientific Officer and cofounder of Inventiva, commented: “We are delighted to have a Fast Track designation for lanifibranor in NASH that extends to the treatment of NASH with compensated cirrhosis, a very severe stage of the disease where patients are in urgent need of treatment. The FDA decision does not only recognize this urgency but also the potential of lanifibranor to address this crucial unmet medical need. This news reinforces our confidence in the unique mechanism of action of our lead drug candidate and confirms our determination to accelerate the development of lanifibranor in its pivotal phase.”
NASH with compensated cirrhosis represents a serious stage of the disease and is becoming the leading cause for liver transplantation, which today represents the only available treatment option for patients who have progressed to end-stage NASH. There is thus an urgent need to develop pharmacological therapies to slow down or halt the progression towards NASH with compensated cirrhosis for NASH patients who are at an increased risk of liver-related morbidity and mortality.
Given the broad range of activity of lanifibranor as a pan-PPAR agonist on multiple features of NASH, including beneficial effects on metabolism, inflammation, ballooning, fibrosis and vascular manifestations, Inventiva believes that there is a strong rationale to evaluate the efficacy of lanifibranor in NASH patients with compensated cirrhosis.
About Fast Track designation
Fast track is a process designed to facilitate the development and expedite the review and potential approval of drug candidates to treat serious, life-threatening conditions and fill an unmet medical need. The purpose is to get important new drugs to the patient earlier.
Determining whether a condition is serious is a matter of judgment, but generally is based on whether the drug will have an impact on factors such as survival, day-to-day functioning, or the likelihood that the condition, if left untreated, will progress from a less severe condition to a more serious one.
Filling an unmet medical need is defined as providing a therapy where none exists or providing a therapy which may be potentially better than available therapy. Any drug being developed to treat or prevent a condition with no current therapy is obviously directed at an unmet need. If there are available therapies, a Fast Track drug must show some advantage over available therapy, which is assessed against a pre-defined set of criteria.
About Inventiva
Inventiva is a clinical-stage biopharmaceutical company focused on the development of oral small molecule therapies for the treatment of NASH, MPS and other diseases with significant unmet medical need.
Leveraging its expertise and experience in the domain of compounds targeting nuclear receptors, transcription factors and epigenetic modulation, Inventiva is currently advancing two clinical candidates, as well as a deep pipeline of preclinical programs.
Lanifibranor, its lead product candidate, is being developed for the treatment of patients with NASH, a common and progressive chronic liver disease for which there are currently no approved therapies. In 2020, Inventiva announced positive topline data from its Phase IIb clinical trial evaluating lanifibranor for the treatment of patients with NASH and obtained both FDA Breakthrough Therapy and Fast Track designation for lanifibranor in the treatment of NASH. Lanifibranor is currently being evaluated in a pivotal Phase III clinical trial.
Inventiva is also developing odiparcil, a second clinical stage asset, for the treatment of patients with subtypes of MPS, a group of rare genetic disorders. Inventiva announced positive topline data from its Phase IIa clinical trial evaluating odiparcil for the treatment of adult MPS VI patients in 2019 and received both FDA Fast Track and Rare Paediatric Disease designation for odiparcil in MPS VI.
In parallel, Inventiva is in the process of selecting an oncology development candidate for its Hippo signalling pathway program. Furthermore, the Company has established a strategic collaboration with AbbVie in the area of autoimmune diseases. AbbVie has started the clinical development of ABBV‑157, a drug candidate for the treatment of moderate to severe psoriasis resulting from its collaboration with Inventiva. This collaboration enables Inventiva to receive milestone payments upon the achievement of pre-clinical, clinical, regulatory and commercial milestones, in addition to royalties on any approved products resulting from the collaboration.
The Company has a scientific team of approximately 70 people with deep expertise in the fields of biology, medicinal and computational chemistry, pharmacokinetics and pharmacology, as well as in clinical development. It also owns an extensive library of approximately 240,000 pharmacologically relevant molecules, approximately 60% of which are proprietary, as well as a wholly‑owned research and development facility.
Inventiva is a public company listed on compartment C of the regulated market of Euronext Paris (ticker: IVA - ISIN: FR0013233012) and on the Nasdaq Global Market in the United States (ticker: IVA). www.inventivapharma.com.
September 21, 2021
https://www.globenewswire.com/