Marius Receives FDA Approval of Kyzatrex for Hypogonadism
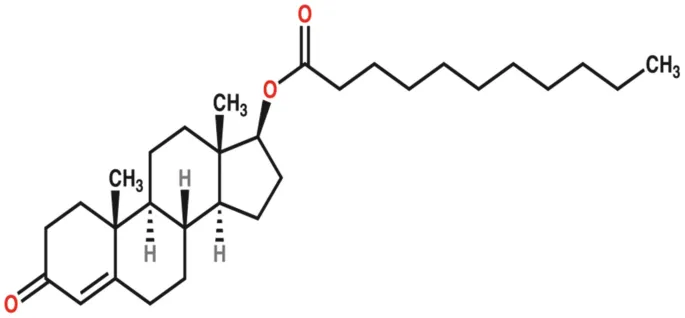
Marius Pharmaceuticals has received FDA’s approval of its Kyzatrex (testosterone undecanoate) for adult male patients with a deficiency or absence of endogenous testosterone, also known as hypogonadism.
The oral softgel capsule is absorbed primarily via the lymphatic system, avoiding liver toxicity. The delivery method avoids the risk of application site reactions with intramuscular testosterone injection and reduces the risk of transference to women or children that can occur with topical testosterone gels and creams.
The approval was supported by results of a late-stage study in which 88 percent of treated participants reached normal levels of testosterone in plasma over 24 hours. The treatment also improved energy levels, erectile function and mood.
The company said that it will continue research into the role of testosterone in male and female health.
About Marius Pharmaceuticals
Marius Pharmaceuticals is paving the way for a new frontier in testosterone. We believe that testosterone is central to overall health and wellbeing, which is why we have spent more than a decade developing a proprietary oral testosterone therapy to help the millions suffering from the unwanted symptoms of testosterone deficiency.
August 9, 2022



