Otsuka Gains Accelerated Approval for Bile Duct Cancer
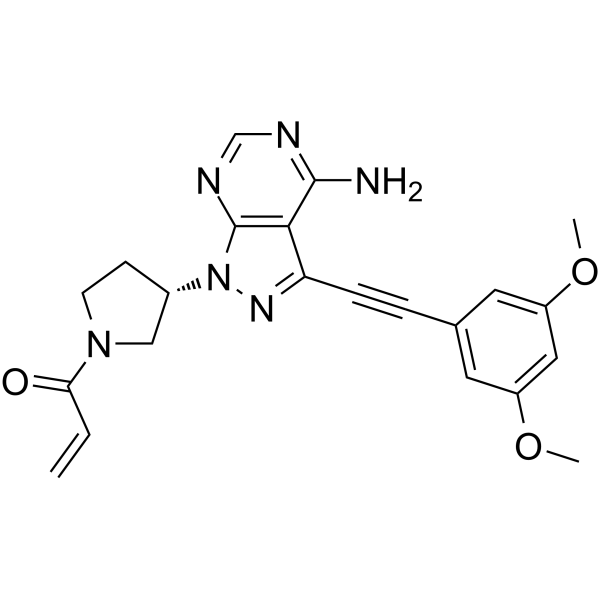
Otsuka subsidiary Taiho Oncology has gained an accelerated approval from the FDA for its Lytgobi (futibatinib) tablets for treatment of adult patients with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma (iCCA) — cancer that occurs in parts of the bile ducts that are inside the liver.
The approval is specifically for patients whose cancers harbor fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements.
The accelerated approval was based on a primary analysis of a phase 2 clinical trial that met its primary endpoint with an objective response rate of 42 percent. The median duration of response was 9.7 months, with 72 percent of responses lasting at least six months.
Continued approval for the drug will depend on the verification of its clinical benefit in a confirmatory trial or trials.
Taiho Oncology, Inc. specializes in the development of orally administered anti-cancer agents and markets these medicines for a range of tumor types in the U.S. Taiho Oncology’s growing pipeline of selectively targeted anti-cancer agents is led by a world-class clinical development organization.
About Taiho Oncology
Taiho Oncology is a subsidiary of Taiho Pharmaceutical Co., Ltd. which is part of Otsuka Holdings Co., Ltd. Taiho Oncology is headquartered in Princeton, New Jersey and oversees its parent company’s European and Canadian operations, which are located in Zug, Switzerland and Oakville, Ontario, Canada. Taiho Oncology is a subsidiary of Taiho Pharmaceutical Co., Ltd. which is part of Otsuka Holdings Co., Ltd. Taiho Oncology is headquartered in Princeton, New Jersey and oversees its parent company’s European and Canadian operations, which are located in Zug, Switzerland and Oakville, Ontario, Canada.
About Taiho Pharmaceutical Co., Ltd.
Taiho Pharmaceutical Co., Ltd. is a Japanese pharmaceutical company, and a subsidiary of Otsuka Holdings, which focuses on developing cancer treatments. Taiho, headquartered in Tokyo, Japan, is an R&D-driven specialty pharma focusing on the three fields of oncology, allergies and immunology, and urology. Taiho also engages in research and development, manufacturing, and the marketing of pharmaceutical products. The company is principally active in the prescription market, but also develops over-the-counter medication, and is a full member of the Japan Pharmaceutical Manufacturers Association (JPMA).
October 4, 2022



