VIVJOA (oteseconazole) to Treat Recurrent Vulvovaginal Candidiasis, USA
VIVJOA is the first and only FDA-approved drug for the treatment of recurrent vulvovaginal candidiasis (RVVC) in females.
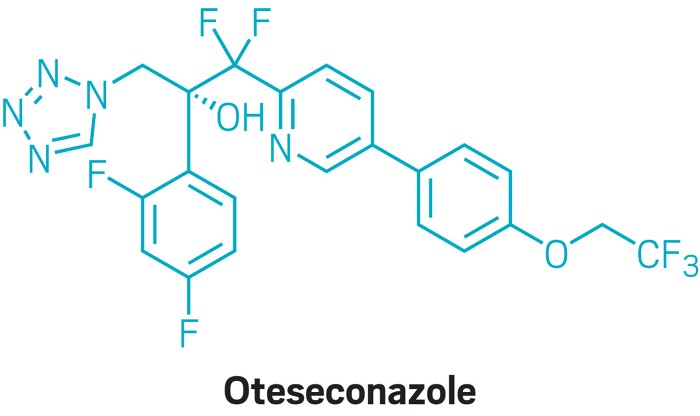
VIVJOA™ is an azole antifungal agent indicated to reduce the incidence of recurrent vulvovaginal candidiasis (RVVC) in females who have a history of RVVC and are not of reproductive potential.
Developed by US-based biopharmaceutical company Mycovia Pharmaceuticals, VIVJOA is available in the form of oral 150mg hard gelatine capsules.
In June 2019, Mycovia and Chinese pharmaceutical company Jiangsu Hengrui Medicine signed an exclusive agreement to develop and commercialise oteseconazole (VT-1161) in China, including Mainland China, Hong Kong, Macau and Taiwan, for either the treatment or prevention of a variety of fungal conditions, including RVVC, onychomycosis, and invasive fungal infections.
In October 2019, Mycovia entered an exclusive license, development and technology transfer agreement with Gedeon Richter, a pharmaceutical company based in Hungary, to commercialise and manufacture VT-1161 for the treatment of RVVC in Europe, Latin America, Australia, Russia, and other Commonwealth of Independent States (CIS) countries.
Regulatory approvals for VIVJOA
In November 2020, Oteseconazole was given the Qualified Infectious Disease Product Status and Fast-Track designation by the US Food and Drug Administration (FDA).
In June 2021, Mycovia submitted a new drug application (NDA) to the FDA for the drug. This was accepted for review by the FDA and granted a six-month priority review in July 2021.
The FDA approved VIVJOA in April 2022.
Recurrent vulvovaginal candidiasis (RVVC) causes and symptoms
Vulvovaginal candidiasis (VVC) is classified into uncomplicated and complicated VVC. It is usually caused by Candida albicans or other Candida species or yeasts.
The most frequent symptoms of the condition are pruritus, vaginal itching, burning, irritation, inflammation and soreness, and external dysuria. Some women may have abnormal vaginal discharge, as well as uncomfortable dyspareunia or urination, which can result in discomfort and pain.
RVVC is defined as three or more occurrences of symptomatic VVC within or less than a year. It affects 9% of women and is caused by repeated antibiotic usage, diabetes, or other underlying host factors. The disease affects an estimated 138 million women worldwide every year.
VIVJOA’s mechanism of action
Oteseconazole is an azole metalloenzyme inhibitor that inhibits the enzyme CYP51 (also known as 14α demethylase). It demethylates the 14-α position of lanosterol to yield ergosterol, a compound that plays a key role in maintaining the integrity of cell membranes in yeast and fungi, thereby inhibiting fungal growth.
Oteseconazole is effective against most microbes associated with RVVC due to its ability to bind and inhibit CYP51. In addition to inhibiting the production of ergosterol, the drug enhances the build-up of 14-methylated sterols, which leads to fungal cell death.
The drug possesses a tetrazole metal-binding group, which decreases its affinity for the human CYP51 isoenzyme and helps minimise off-target toxicity.
Clinical trials on VIVJOA
The FDA’s approval of VIVJOA was based on results from three Phase III clinical trials. These were two global, pivotal studies named VIOLET and a US-focused study named ultraVIOLET.
The VIOLET studies were initiated in 2018 to evaluate the safety and efficacy of oteseconazole (VT-1161) in patients with RVVC. A total of 656 patients were enrolled in the two studies, which included a maintenance and induction phase.
The first trial enrolled 483 patients in the induction phase, 326 of whom were enrolled in the 48-week maintenance phase. A total of 217 patients were randomised to receive oteseconazole, while the rest were randomised to receive placebo.
For the second trial, 425 patients were enrolled in the induction phase, 330 of whom were moved to the 48-week maintenance phase. A total of 220 patients were randomised to receive oteseconazole, with the remaining patients being given placebo at random.
The two trials showed, respectively, that 93.3% and 96.1% of women with RVVC who received oteseconazole did not have a recurrence of infection during the maintenance phase, compared with 57.2% and 60.6% of patients who received placebo.
The ultraVIOLET study was conducted to compare oteseconazole’s safety and efficacy with that of fluconazole over a period of 50 weeks. A total of 220 patients were enrolled in the study across 51 sites.
The first part of the study was a two-week treatment period with either fluconazole or oteseconazole. The second part was a 11-week treatment period with either oteseconazole or a placebo, and then a 37-week follow-up period. Oteseconazole demonstrated strong activity against the Candida glabrata species of yeast, which are often resistant to fluconazole.
Results from the ultraVIOLET study showed that in 89.7% of women with RVVC who were administered oteseconazole, the yeast infection cleared and did not recur for the 50-week maintenance period, compared with 57.1% in the fluconazole group.
The most common side effects in the clinical studies were headache, nausea and diarrhoea.
Other Phase III clinical trials on Vivjoa
In April 2021, Jiangsu Hengrui completed the SHR8008-302 Phase III study of oteseconazole, which compared the drug with fluconazole for the treatment of acute VVC.
Another Phase III clinical study, SHR8008-301, began in September 2021. This trial aims to evaluate and compare the efficacy of oteseconazole with fluconazole in the treatment of RVVC in women.
The results of the studies are expected to help when submitting an NDA for the drug in China.
4 August 2022



