PepGen earns FDA Fast Track Designation for myotonic dystrophy type 1 candidate
A Phase I trial studying the therapy is underway after an FDA clinical hold was lifted last year, with initial data expected in 2024.
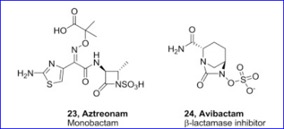
The US Food and Drug Administration (FDA) has granted Fast Track Designation to PepGen’s candidate for the treatment of myotonic dystrophy type 1 (DM1).
The US-based biotech is now able to meet with the agency more frequently to discuss the drug’s development and is eligible for accelerated approval and priority review. It will also be able to submit parts of an application for approval in stages in a rolling review, as per fast track designation benefits.
PepGen’s candidate PGN-EDODM1, which was granted FDA Orphan Drug Designation in September 2023, delivers a peptide-conjugated antisense oligonucleotide (ASO) to cells. It targets the RNA processing factor MBNL1, a protein that is sequestered in DM1 patients.
Through this therapy, PepGen aims to restore MBNL1, thereby stopping downstream mis-splicing events and the expression of dysfunctional proteins important in the musculoskeletal system, the biotech said in a 20 February press release.
DM1 patients usually have stiff and weak muscles, along with cardiac and respiratory issues. Also known as Steinert’s disease, the condition is estimated to affect 40,000 people in the US.
PepGen is evaluating its candidate in the Phase I FREEDOM-DM1 clinical trial. The randomised trial is estimated to enrol 24 participants with DM1, who will receive PGN-EDODM1 at three dose levels. The primary outcome is measuring the number of participants with adverse events and other abnormal parameters after 16 weeks. Initial data from the study is expected by the company this year.
The trial has had to overcome a stumbling block when the FDA placed it on a clinical hold.
Also in the DM1 clinical space is Dyne Therapeutics’ orphan drug designated DYNE-101. Dyne has already reported positive results from a Phase I/II trial assessing the candidate in DM1 patients.
PepGen is also developing PGN-EDO51 for patients with exon 51 amenable Duchenne muscular dystrophy. The candidate is being assessed in the Phase II CONNECT1-EDO51 study. The first patient was dosed in January 2024, with preliminary data expected in mid-2024.
The publicly listed biotech announced cash and equivalents of $129.5m when announcing Q3 results in September 2023. The company’s cash runway is expected to flow into 2025.
February 23, 2024