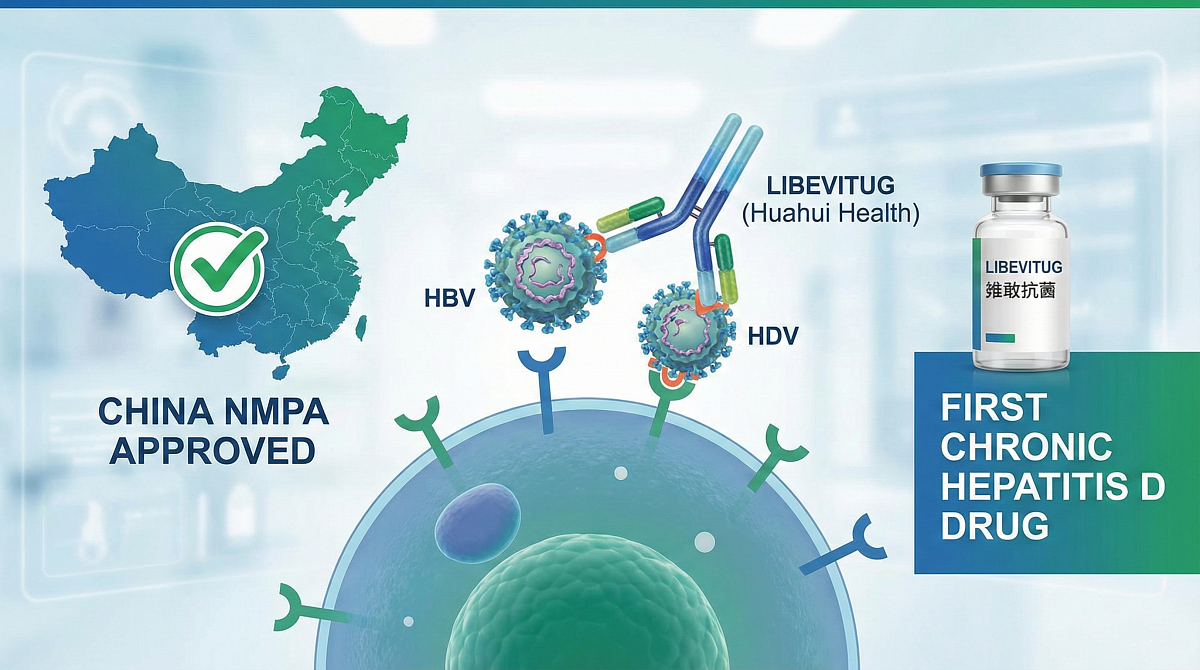
China Clears Huahui Health’s Libevitug as First Drug for Chronic Hepatitis D
- Drug: Libevitug
- Company: Huahui Health
- Regulator: NMPA (China)
- Indication: Chronic HDV
- Mechanism: Entry Inhibitor
- Target: PreS1 Domain
Huahui Health has won the first approval in China for a drug to treat chronic infections with hepatitis D virus (HDV), plugging a major gap in treatment options.
China's National Medical Products Administration (NMPA) has granted conditional approval for libevitug, an antibody targeting the PreS1 domain of the large envelope protein of hepatitis B virus (HBV) and HDV, blocking entry of the viruses into liver cells.
Comparison to Existing Treatments
To date, the only other drug approved for HDV is Gilead Sciences' Hepcludex (bulevirtide), another entry inhibitor that has been approved in Europe in 2023, but was rejected by the FDA in the US.
At the moment, the go-to therapy for HDV and HBV in China is off-label treatment with pegylated interferon alpha, which has limited efficacy and can cause significant side effects.
HDV Burden and Health Risks
HDV is a virus that exists only as a co-infection with HBV and is seen in around 5% of the estimated 254 million people around the world with chronic hepatitis B, equivalent to around 13 million cases. Like HBV, HDV is most commonly transmitted from mother to child during birth, and through contact with blood or other body fluids.
Having both HBV and HDV increases the risk of liver disease-related death and liver cancer, and mortality rates can be as high as 50% within five years.
China's Prevalence Landscape
China has the world's highest burden of chronic HBV infection, with an estimated 75 million people harbouring the virus, accounting for roughly one-third of the global total population. A study published in the Journal of Clinical and Translational Hepatology last year found the prevalence of HDV antibodies was, however, quite low at 0.24%.
International Studies and Future Outlook
The company is also testing the drug in international studies involving the US, Pakistan, and Mongolia.
WHO Guidelines
The WHO's current guidelines for the prevention, diagnosis, care, and treatment of people with chronic HBV recommend that serological testing be carried out for anti-HDV antibodies in all those who test positive for hepatitis B surface antigen (HBsAg), so their elevated risk of serious complications can be anticipated and managed.