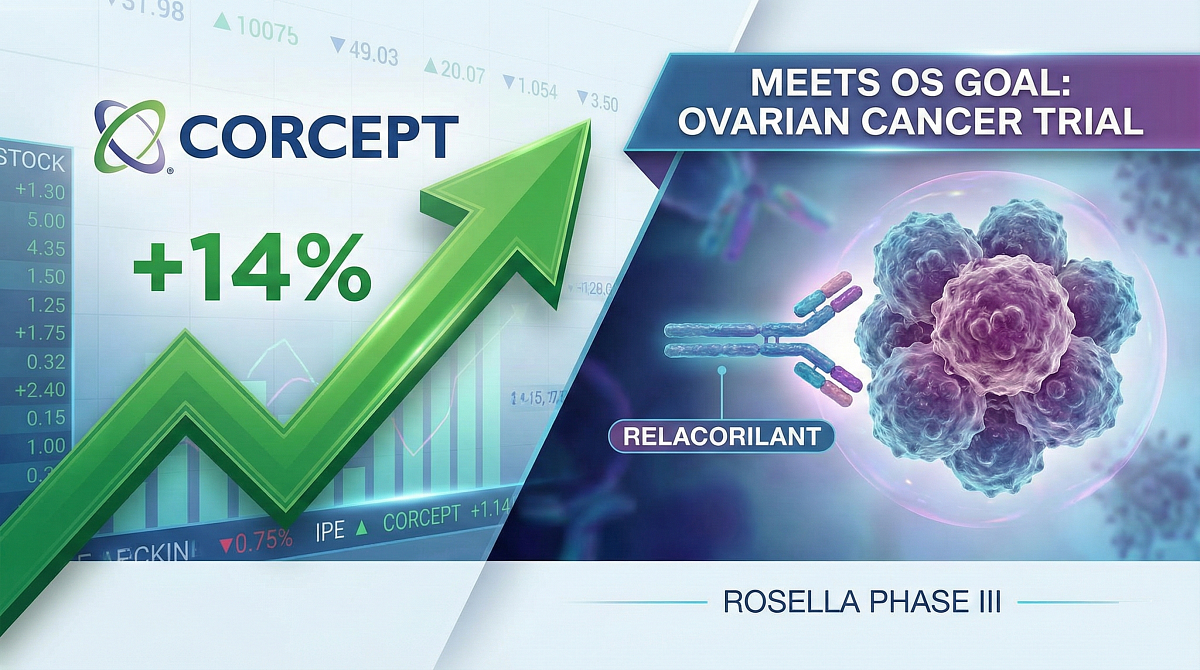
Corcept’s Stock Up as Relacorilant Meets OS Goal in Phase III Ovarian Cancer Trial
- Stock Rise: +14.6%
- New Price: $41.62
- Market Cap: $4.36bn
- Median OS Benefit: +4.1 Months
Corcept Therapeutics’ stock has risen 14% after its selective cortisol modulator relacorilant (developmental code name CORT-125134) met its overall survival (OS) goal in a Phase III trial in ovarian cancer.
The company’s stock rose 14.6% after the endpoint success was announced, from $36.31 at market close on 21 January to $41.62 at market open on 22 January. The company has a market cap of $4.36bn.
ROSELLA Study Results (NCT05257408)
In the ROSELLA study, which enrolled 381 patients with platinum-resistant ovarian cancer, patients treated with relacorilant plus nab-paclitaxel experienced a 35% reduction in the risk of death compared to patients treated with nab-paclitaxel alone.
The median OS for patients receiving relacorilant was 16.0 months, compared to 11.9 months for patients receiving nab-paclitaxel alone, a clinically meaningful difference of 4.1 months.
Previously, in April 2025, Corcept announced that ROSELLA met its primary endpoint of improved progression-free survival (PFS), with a 30% reduction in the risk of disease progression between the two cohorts.
— Dr Alexander Olawaiye, Magee-Women’s Hospital, University of Pittsburgh
Safety and Tolerability Profile
Relacorilant in combination with nab-paclitaxel was well-tolerated, consistent with its known safety profile. The frequency and severity of adverse events (AEs) in the combination arm were comparable to those in the nab-paclitaxel monotherapy arm.
Pipeline Context and FDA Status
Corcept recently suffered a blow after relacorilant was rejected by the FDA for use in Cushing’s syndrome. The company suffered a stock hit at the end of December 2025 after receiving a complete response letter (CRL) from the US Food and Drug Agency (FDA). While the agency agreed that both the GRACE and GRADIENT trials supported the filing, the agency required additional evidence of effectiveness to balance out the benefit-risk ratio.
Complete results from ROSELLA will be presented at an upcoming medical conference. Corcept is currently studying relacorilant, which is taken orally, in other solid tumours, including platinum-sensitive ovarian, endometrial, cervical, pancreatic and prostate cancers.