FDA Approves Mitapivat: First Oral Pill for Thalassemia
First Oral Pill For Adults With Thalassemia Anaemia Gets Approval, Experts Hail It As Game Changer
The US Food and Drug Administration (FDA) has granted approval for the first-ever oral treatment for anaemia in adults with thalassemia. This historic milestone is being hailed by global health experts as a transformative "game changer" in the management of this complex genetic blood disorder.
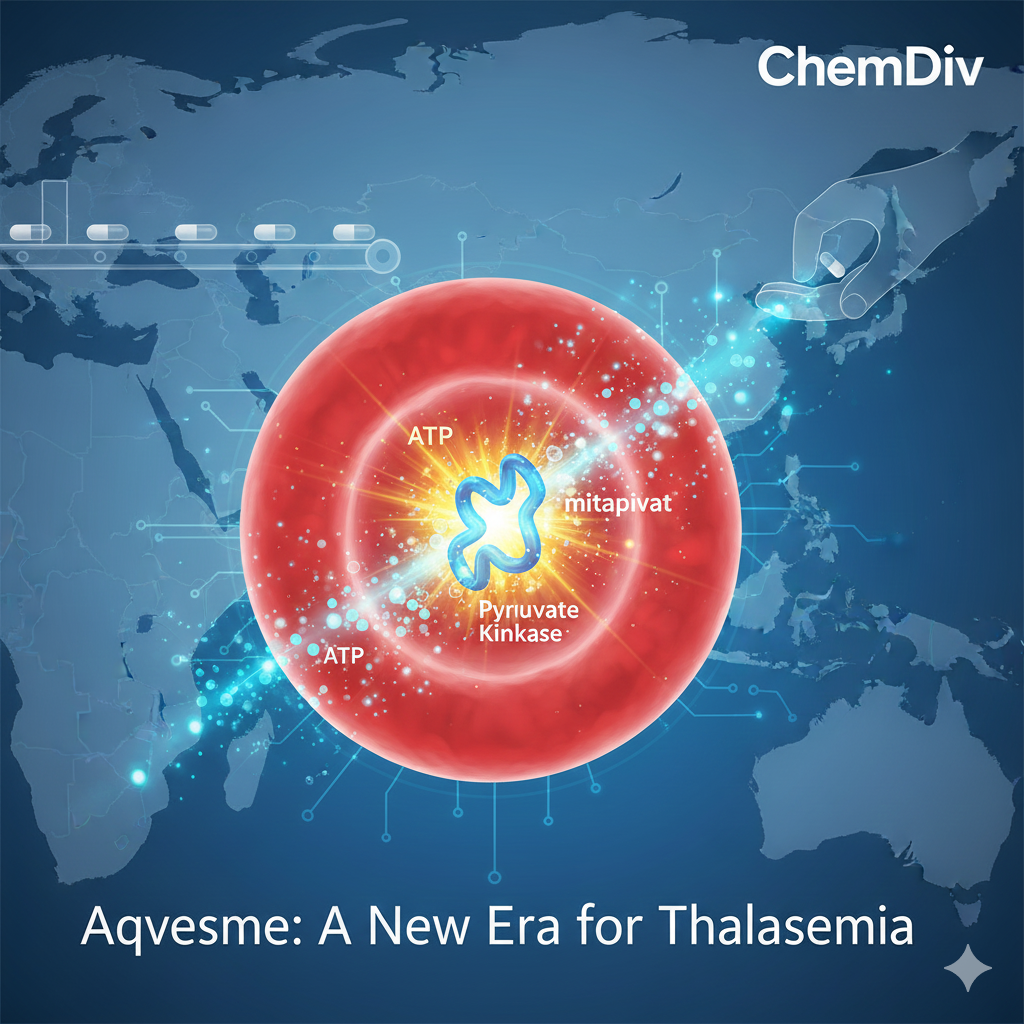
The newly approved drug, mitapivat (to be marketed under the brand name Aqvesme), is indicated for the treatment of anaemia in adults with both alpha- and beta-thalassemia. This represents a paradigm shift; for decades, patients have been tethered to a grueling regimen of lifelong blood transfusions and chelation therapy. Mitapivat stands as the first medication capable of addressing both transfusion-dependent (TDT) and non-transfusion-dependent (NTDT) forms of these inherited conditions.
The Science Behind Mitapivat: Activating Pyruvate Kinase
Mitapivat is a first-in-class pyruvate kinase activator. To understand its impact, one must look at the cellular core of the disease. In patients with thalassemia, red blood cells are metabolicially fragile, breaking down prematurely and causing chronic anaemia. Mitapivat directly targets this metabolic pathway, enhancing the energy balance and cellular ATP production within red blood cells.
By boosting this energy production, the medication allows red blood cells to survive significantly longer and function more effectively in the bloodstream. Clinical data suggests that as red blood cell health improves, haemoglobin levels rise, leading to a marked decline in the frequency and necessity of blood transfusions.
Clinical Benefits of Oral Therapy:
- ✓ Reduced Treatment Burden: Transitioning from clinical infusions to a single daily oral pill.
- ✓ Mitigating Complications: Potential to lower long-term risks associated with iron overload from frequent transfusions.
- ✓ Patient Quality of Life: Significant reduction in fatigue and chronic exhaustion linked to severe anaemia.
Global Impact: Hope for the "Thalassemia Capital"
The approval resonates particularly strongly in India, often referred to as the "thalassemia capital of the world." With every eighth thalassemia patient globally residing in India, the burden on the national blood supply and healthcare infrastructure is immense. Haematologist Dr. Rahul Bhargava emphasizes that this drug addresses the cellular core of the disease rather than merely managing its secondary consequences.
“Mitapivat can be a big path-breaking drug,” notes Dr. Satyam Arora of PGICH, Noida. “Managing patients with a single oral pill will help us optimize blood bank resources more efficiently across the country.” The medical community is now calling for expeditious access to ensure this innovation reaches various categories of patients who have historically had limited sustainable treatment options.
Conclusion: A Sustainable Future in Blood Disorder Management
While bone marrow transplantation remains a curative option, it is not accessible or suitable for all. The introduction of mitapivat (also sold under the name Pyrukynd) offers a new ray of hope for sustainable, patient-centered care. By improving red blood cell survival, it may even delay or prevent the progression to transfusion dependence in certain non-transfusion-dependent groups, fundamentally altering the trajectory of the disease.



