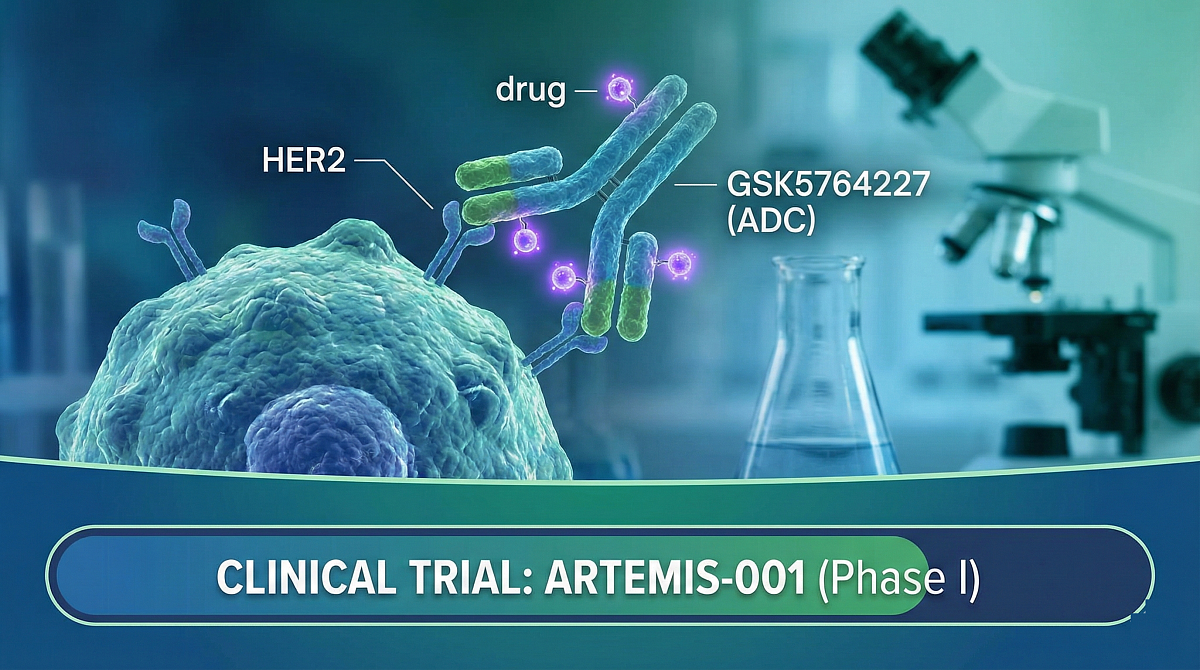
GSK5764227 (HS-20093): New HER2-Targeted ADC Treatment for Cancer
- Drug Name: GSK5764227
- Alias: HS-20093
- Type: Antibody-Drug Conjugate
- Target: HER2 Protein
- Trial: ARTEMIS-001 (Phase I)
- Status: Experimental
GSK5764227, also known as HS-20093, is an experimental cancer medicine currently being studied in people whose tumors have a protein called HER2. This treatment is still in early clinical trials and is not yet approved. Researchers are studying it to understand how safe it is, how it works in the body, and whether it may help control cancer.
Why HER2 Is Important In Cancer
HER2 is a protein found on the surface of some cells. In certain cancers, HER2 is present in higher amounts or sends abnormal growth signals. This can cause cancer cells to grow and divide more quickly than normal. It is commonly involved in certain breast cancers, stomach cancers, lung cancers, and other solid tumors. Because of this, HER2 has become an important target for cancer treatment.
How GSK5764227 (ADC) Works
GSK5764227 belongs to a group of medicines called antibody–drug conjugates, often referred to as ADCs. This type of treatment combines two components into a single medicine:
- An antibody: Recognizes and attaches to HER2 on cancer cells.
- Cancer-killing medicine: A small amount of a drug component.
After the drug attaches to a HER2-positive cancer cell, it is taken inside the cell. Once inside, the cancer-killing component is released, which may help destroy the cancer cell from within. This design aims to deliver treatment more directly to cancer cells while limiting exposure to healthy tissue.
The ARTEMIS-001 Clinical Study
GSK5764227 is being tested in a clinical trial called ARTEMIS-001. This is a Phase I study, meaning it is the first time this medicine is being given to people. The main goals are to determine how safe the drug is, identify the most appropriate dose, and understand how the body processes the treatment.
Patients enrolled in this study typically have advanced cancer and have already tried other available treatments. Researchers also look for early signs that the medicine may help control cancer, although proving effectiveness is not the primary goal at this stage.
Observations and Safety
So far, doctors have observed that side effects are generally manageable. Most side effects reported have been mild to moderate, including fatigue, digestive issues, changes in blood counts, and lung-related symptoms. Doctors may adjust the dose or provide supportive treatments to help patients continue therapy safely. Researchers have also seen early signs that the medicine is biologically active in some HER2-positive cancers.
Key Takeaway for Patients
GSK5764227 represents a new approach being explored for people with HER2-positive cancers, particularly for those whose disease has progressed after other HER2-targeted therapies. While experimental, early research suggests it may become another option in the future if ongoing studies continue to show benefit and safety.