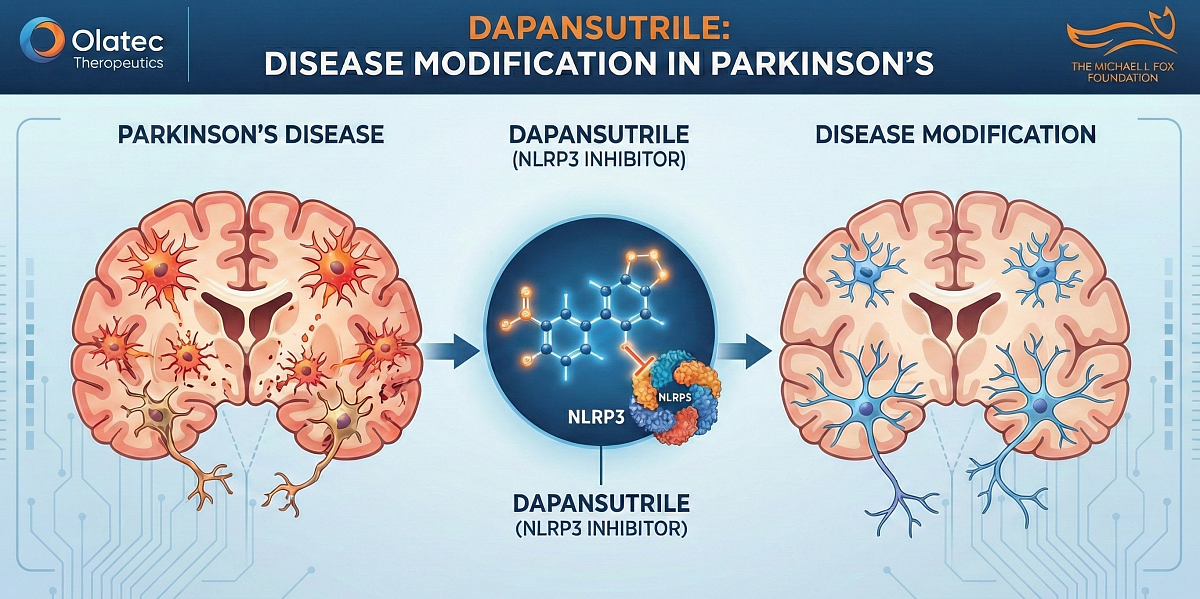
Olatec Therapeutics Presented Preclinical Evidence of Disease-Modifying Activity with Dapansutrile in its Parkinson's Studies
- Drug: Dapansutrile (NLRP3 inhibitor)
- Event: MJFF Research Exchange
- Result: Broad, durable disease-modifying effects
- Next Step: Phase 2 Trial (Cambridge)
Olatec Therapeutics, Inc., a clinical stage biotechnology company leading the development of specific NLRP3 inhibitors, presented preclinical data at The Michael J. Fox Foundation (MJFF) Parkinson's Disease Research Exchange forum.
The studies were supported by funding from MJFF for Parkinson's Research and conducted by Dr. Nadia Stefanova of the Medical University of Innsbruck. The results presented by Dr. Stefanova demonstrated that chronic oral treatment with dapansutrile produces broad, durable, and clinically relevant disease-modifying effects in two translational mouse models of Parkinson's disease (PD).
Preclinical Efficacy and Biomarker Data
Dr. Stefanova's preclinical studies showed dapansutrile, which was orally administered for six months at a well-tolerated human-equivalent dose:
- Inhibited neuroinflammation (microglial and astroglial activation)
- Prevented or significantly improved motor deficits
- Protected dopaminergic neurons in the substantia nigra and striatum
- Reduced α-synuclein pathology and intracellular accumulation
- Reversed pathogenic microglial gene-expression signatures, normalizing ~75% of upregulated genes (including Lrrk2) and counteracting the disease-associated microglial phenotype identified by human single-cell RNA sequencing
- Lowered plasma neurofilament light (NfL) and IL-18, biomarkers that correlated strongly with motor improvement
Mechanism of Action and Models
These effects were observed and target engagement confirmed (reduced NLRP3, ASC, cleaved caspase-1, IL-1β, IL-18) in the brain in two models: a PD prevention model (i.e., α-synuclein pre-formed fibril model) and, critically, in a PD therapeutic model initiated after symptom onset (i.e., PLP-α-synuclein transgenic model).
— Dr. Nadia Stefanova, Medical University of Innsbruck
Future Clinical Development: Phase 2 Trial
Damaris Skouras, Co-Founder and CEO of Olatec, stated: "We are grateful to MJFF and Dr. Stefanova for enabling these studies that provide compelling mechanistic and biomarker evidence that dapansutrile can modify core neurodegenerative processes in animal models of PD."
The data further strengthen the translational rationale for dapansutrile's Phase 2 trial expected to begin enrolling soon at the University of Cambridge. If confirmed in patients who will be dosed for 12 months, dapansutrile could represent the first oral disease-modifying therapy targeting NLRP3-driven neuroinflammation in Parkinson's.