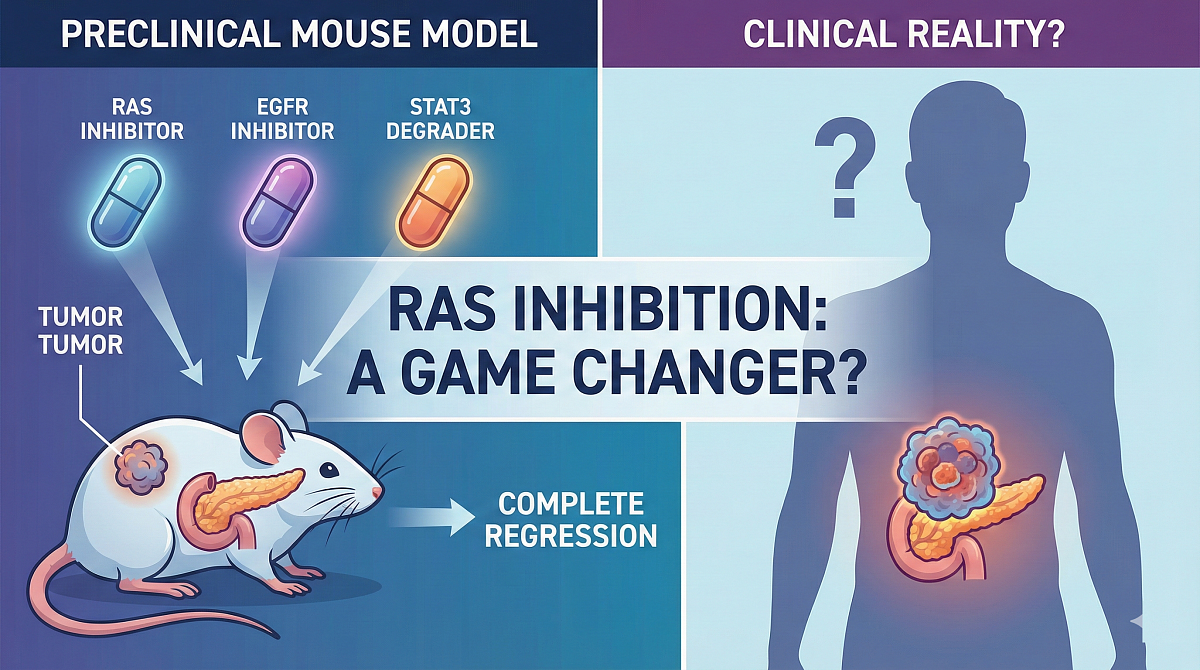
RAS Inhibition: A Game Changer in Pancreatic Cancer?
- Lead: CNIO (Madrid)
- Target: Raf1, Egfr, Stat3
- Therapy: Triple Combination
- Inhibitors: Daraxonrasib, Afatinib, SD36
- Result: Complete Regression
- Status: Mouse Models Only
Preclinical findings show antitumour activity of triple KRAS inhibition in mouse models, but it will take years of rigorous research to assess its clinical efficacy in oncology patients.
— Dr. Rachna T. Shroff, University of Arizona Cancer Center
Dr. Shroff's experience was shared by many other oncology professionals after a preclinical research study published in Proceedings of the National Academy of Sciences (PNAS) hit the news globally.
The CNIO Study: Triple-Combination Therapy
The team of the Experimental Oncology Group at the National Cancer Research Centre (CNIO) in Madrid, Spain, led by Prof. Mariano Barbacid, investigated the antitumour effect of targeting three nodes that control KRAS signalling pathways — Raf1, Egfr, and Stat3.
They applied a triple-combination therapy of:
- Daraxonrasib: A novel RAS(ON) inhibitor.
- Afatinib: An EGFR-tyrosine kinase inhibitor (TKI).
- SD36: A selective proteolysis targeting chimera (PROTAC) degrader of STAT3 protein.
The study was conducted in vitro and in genetically engineered mouse (GEM) models of pancreatic ductal adenocarcinoma (PDAC). Tumour-bearing mice treated with the novel treatment underwent rapid apoptotic cell death that resulted in complete tumour regressions and remained tumour-free for over 200 days post-treatment, suggesting a potential therapeutic impact overcoming the onset of tumour resistance.
From Preclinical Success to Clinical Reality
These are exciting news, since the results may represent a significant step toward understanding therapeutic vulnerabilities of pancreatic cancer. But they are not yet applicable to patient care.
— Dr. Ben Westphalen, Chair, ESMO Precision Oncology Task Force
Dr. Westphalen specifically recalls the case of the Hedgehog cellular signalling pathway, whose inhibition appeared to be a promising strategy in mouse models of PDAC years ago. However, subsequent research did not confirm those preclinical findings in phase I studies.
Challenges: Toxicity and Resistance
What needs to be proven is not only that the novel triple combination therapy is effective in people with PDAC, but also what potential side-effects it may carry in humans. Dr. Shroff warns, “We already know that the combination of EGFR and KRAS targeting agents alone has overlapping toxicities, and the addition of the STAT3 PROTAC may introduce further adverse effects.”
Current Landscape and Unmet Needs
The excitement surrounding the Spanish study findings is partly driven by the dismal prognosis of PDAC. Patients' treatment relies today on gemcitabine, either alone or in combination with nab-paclitaxel, and 5-fluoracil (F) based regimens such as FOLFIRINOX and nalIRIFOX with limited benefits.
However, toxicity and primary or acquired resistance are major challenges to the clinical use of these targeted therapies. While hope for innovative and effective RAS-inhibitors is high, whether RAS inhibition will be a true breakthrough in this difficult-to-treat disease is uncertain.