Clinical Trial Launched to Test Experimental Intranasal Spray Against Respiratory Viruses
- Lead: UMSOM (CVD)
- Therapy: INNA-051
- Target: Healthy Adults (18-45)
- Enrollment: 1,100 Participants
- Mechanism: TLR2/6 Agonist
A new clinical trial led by researchers at the University of Maryland School of Medicine's (UMSOM) Center for Vaccine Development and Global Health (CVD) will test a new experimental intranasal spray designed to boost immune defenses and reduce illness from respiratory viruses.
Last year, at least one million people in the U.S. were hospitalized for respiratory virus illnesses like the flu or COVID-19. Many of these individuals were at higher risk of getting infections due to living or working around young children who contract more respiratory infections.
Phase 2 Study Design and Enrollment
The study is being conducted in collaboration with ENA Respiratory, the manufacturer of the novel therapy. The randomized double-blind Phase 2 trial aims to enroll 1,100 healthy adults ages 18 to 45 years who are at increased risk of upper respiratory virus infections due to exposure to young children or frequent close contact with others.
Participants will be randomly assigned to receive the intranasal spray, called INNA-051, or a placebo spray to determine if INNA-051 is safe and works better than the placebo at boosting the immune response and preventing illness.
Mechanism: TLR2/6 Agonist
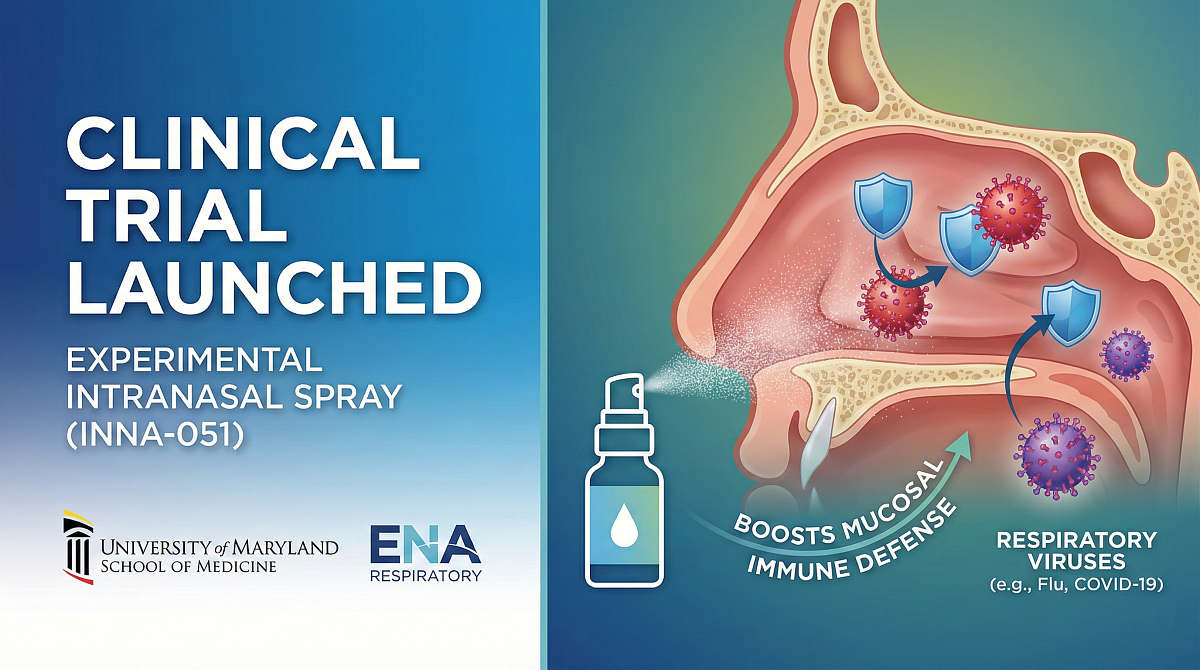
A non-vaccine, intranasal spray, INNA-051 works as a prophylactic drug designed to be taken weekly during cold and flu season. It is a TLR2/6 agonist, which works by priming the immune system's first line of defense in an effort to accelerate the clearance of harmful germs from nasal passages.
INNA-051 is virus-agnostic, meaning that it can potentially help protect against a wide array of viruses, and acts in the nasal passages, the site of initial replication of common respiratory viruses.
Expert Perspectives
— Justin Ortiz, MD, UMSOM Principal Investigator
UMSOM Dean Mark T. Gladwin, MD, added that researchers are aiming to demonstrate the exciting potential of TLR2/6 agonists to become the first prophylactic therapy against respiratory viral illness, strengthening the mucosal surfaces of airways.
James Campbell, MD, MS, noted that INNA-051 has the potential to protect those most vulnerable to respiratory viral complications, including persons with chronic lung disease, heart disease, and diabetes.