PLpro Library
ChemDiv’s library of small molecules specifically inhibiting papain-like protease contains 6,442 compounds.
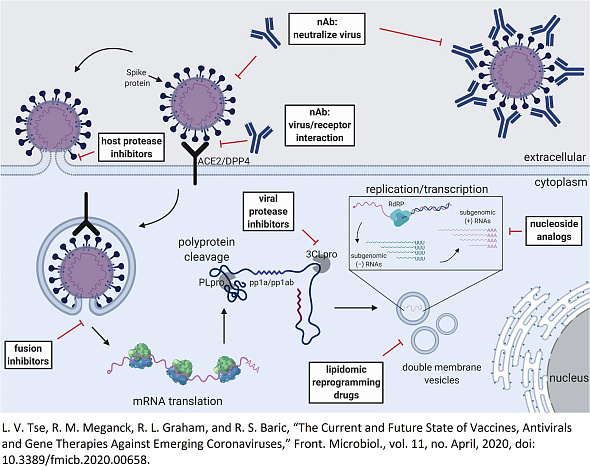
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a virus classified within the betacoronavirus genus. As a characteristic feature of betacoronaviruses, SARS-CoV-2 encodes a papain-like protease (PLpro), which plays a crucial role in the cleavage of the viral polypeptide, presenting an essential step in the life cycle of the virus.
PLpro represents an attractive target for antiviral drug development due to its indispensable role in coronaviral replication. Additionally, PLpro contributes to the suppression of host innate immune responses. It achieves this through the reversal of post-translational modifications of host proteins, specifically ubiquitination and ISG15-related modifications. Consequently, targeting PLpro with antiviral drugs could not only block viral replication but also mitigate the dysregulation of signaling pathways in infected cells. This dysregulation often leads to the death of surrounding, uninfected cells.
Structural analyses have revealed that the flexibility of the BL2 loop, located near the active site of PLpro, and the specific amino acid side chains within this loop, significantly impact the efficacy of inhibitors. These findings underscore the importance of considering structural differences and the dynamic nature of the BL2 loop in the design of inhibitors, which were implemented in designing our library of PLpro inhibitors. Those compounds comprised by our library would be effective against SARS-CoV-2 PLpro and potentially other coronavirus PLpro enzymes, offering a strategic approach in the fight against COVID-19 and related coronaviral infections [1, 2].
The ChemDiv’s PLpro-specific library, tailored for targeting the papain-like protease of SARS-CoV-2, holds immense potential in antiviral drug discovery. By providing a focused collection of compounds that inhibit PLpro activity, this library can expedite the identification of candidates capable of disrupting key viral replication mechanisms. Additionally, our library helps explore the nuances of PLpro interaction with potential inhibitors, thereby facilitating the development of drugs that can effectively attenuate the virus's ability to suppress the host's immune response. This targeted approach not only streamlines the drug discovery process but also enhances the likelihood of finding potent antivirals against SARS-CoV-2 and potentially other coronaviruses with similar protease characteristics.
References
[1] B. T. Freitas et al., “Characterization and Noncovalent Inhibition of the Deubiquitinase and deISGylase Activity of SARS-CoV-2 Papain-Like Protease,” ACS Infect. Dis., Jun. 2020, doi: 10.1021/acsinfecdis.0c00168.
[2] Y. M. Báez-Santos, S. E. St. John, and A. D. Mesecar, “The SARS-coronavirus papain-like protease: Structure, function and inhibition by designed antiviral compounds,” Antiviral Res., vol. 115, pp. 21–38, 2015, doi: 10.1016/j.antiviral.2014.12.015.
Related