DMSO

Applications of DMSO
Dimethyl sulfoxide (DMSO) is an organic chemical polar compound in a liquid state under normal conditions. It is the aprotic solvent or the solvent which, when decomposed in solution, does not form a free hydrogen ion (proton). The substance is a sick liquid with no color and with a specific smell. Compound is miscible with water, ethanol, chloroform, and benzene in all proportions. It is also highly soluble in diethyl alcohol and acetone. In industry, dimethyl sulfoxide is produced by catalytic oxidation of dimethyl sulfide (DMS) with oxygen or by oxidation with nitrogen dioxide [1].

Figure 1. Graphical representation of sulfide oxidation [1].
Other physical and chemical properties of DMSO include boiling temperature at 189 degrees Celsius (Table 1) and low volatility. These two facts determine the possibility of dimethyl sulfoxide usage as a solvent. Moreover, these days the compound has a plenty of other applications that will be considered in the article.
DMSO as a solvent
DMSO has been generally used as a commercial solvent in pharma industry for more than six decades. Multiple specific chemical reactions are more effective if done in DMSO solution, which results in higher product yield. The powerful soluble properties of the compound allow preparing highly concentrated components in agrochemical formulations.
High boiling point (Table 1) allows DMSO to have a slow evaporation speed. This property makes it convenient to use the compound as a solvent in the chemical reactions that require heating. The high freezing point (around 18 degrees Celsius, Table 1), on the other hand, can limit its applications in a number of chemical processes, although it means that DMSO can be stored at room temperature without any problem.
Table 1. DMSO properties [2]
|
Boiling point |
189 oC (372 oF) |
Freezing point |
18.5 oC (65.3 oF) |
|
Acidity |
(pKa) 35 |
Polarity |
Polar aprotic |
|
Density |
1.004 g/mL |
Water solubility |
Soluble |
|
Appearance |
Colorless liquid |
LD50 |
14,500 mg/kg |
High solvent polarity of dimethyl sulfoxide makes it easier to dissolve other polar and non-polar compounds in it. Of course, there are other solvents with similar characteristics (such as high boiling point or stabilization of charged intermediates): dimethylacetamide, dimethylformamide, HMPT and N-methyl-2-pyrrolidone are the examples. However, all else being equal, DMSO is significantly less toxic. Lethal doses of dimethyl sulfoxide administrated topically, orally or parenterally to laboratory animals are high [3].
Like any other solvent, dimethyl sulfoxide has its own limitations of usage. Nowadays, solubility in DMSO is one of the parameters required for high-throughput screening (HTS) assays. Machine learning methods were used for classification of molecules as soluble and non-soluble in DMSO [4]. One of the reasons for DMSO having difficulties as a solvent is because of not being inert. This compound in several specific conditions becomes a part of the chemical reaction itself – and people have found applications in this fact!
DMSO applications in research
Dimethyl sulfoxide, as it was stated previously, can be an active participant in chemical reactions, thus it is used in chemical and biological research. The compound is known to regulate numerous molecular mechanisms in cell: DNA replication, recombination and gene expression regulation are one of the most popular ones [5]. Therefore, it is used in polymerase chain reaction (PCR) to enhance PCR amplification by preventing reannealing of denatured DNA so that primers can bind at their correct complementary locations.
Another well-known approach for DMSO in scientific research is as a cryoprotectant for cell cultures. As a result of dimethyl sulfoxide presence in the cell freezing medium, intracellular and extracellular crystals of ice do not grow throughout the cell freezing process. That prevents destruction and death of cells and is usually used for cell storage.
DMSO as a drug
Research for dimethyl sulfoxide as a potential drug has begun in 1961, when dr. Stanley W. Jacob, MD, discovered its ability to penetrate through cell membranes with little to no resistance. The discovery was followed by further studies of the drug that showed an ability of DMSO to carry other molecules through membranes. That fact made the compound one of the possible drug delivery systems.
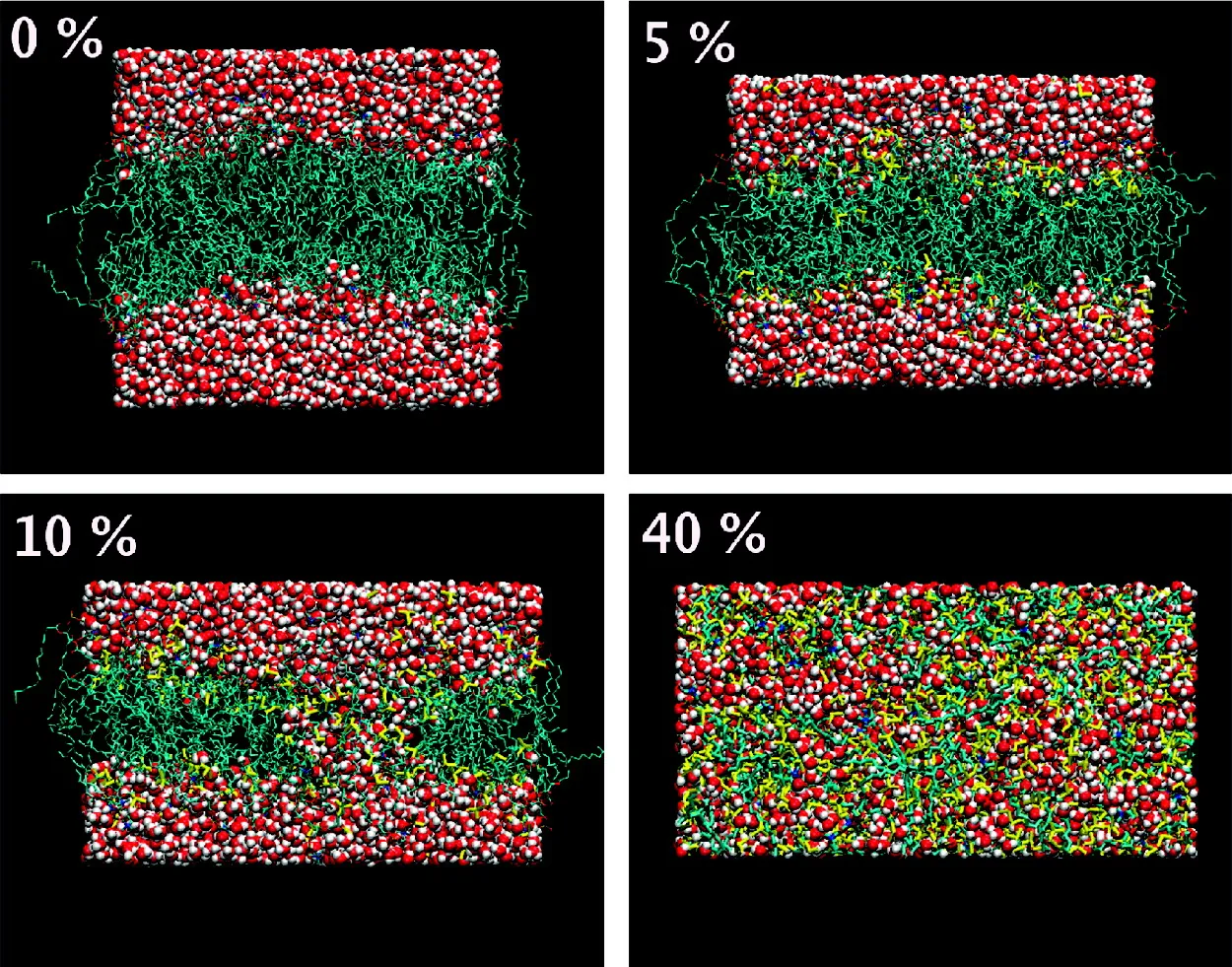
Figure 1. Distinct modes of action of dimethyl sulfoxide on phospholipid membranes depending on % of DMSO (lipid-free basis). Lipids are shown in cyan color, water in red color, and DMSO in yellow color. [6]
Since then studies on DMSO as a treatment for various diseases were conducted. Nowadays, Food and Drug Administration (FDA) approved the drug only as a preservative of organs for transplant and for interstitial cystitis. However, scientific research has shown its applications in pain reduction, dentistry, oncology, virology, dermatovenereology, urology, etc. [7]
To conclude, DMSO is a good solvent with plenty of other uses. ChemDiv’s chemical compounds are dissolved in DMSO to be delivered, providing safe and long-term storage.
References
-
Kupwade, R. (2019). A Concise Review on Synthesis of Sulfoxides and Sulfones with Special Reference to Oxidation of Sulfides. Journal of Chemical Reviews. 1.
-
Xiang, J.-C., Gao, Q.-H., & Wu, A.-X. (2017). The Applications of DMSO. Solvents as Reagents in Organic Synthesis, 315–353.
-
Brayton, C. F. (1986). Dimethyl sulfoxide (DMSO): a review. The Cornell veterinarian, 76(1), 61–90.
-
Tetko, I. V., Novotarskyi, S., Sushko, I., Ivanov, V., Petrenko, A. E., Dieden, R., … Mathieu, B. (2013). Development of Dimethyl Sulfoxide Solubility Models Using 163 000 Molecules: Using a Domain Applicability Metric to Select More Reliable Predictions. Journal of Chemical Information and Modeling, 53(8), 1990–2000.
-
Lv, B., Dai, Y., Liu, J., Zhuge, Q., & Li, D. (2015). The Effect of Dimethyl Sulfoxide on Supercoiled DNA Relaxation Catalyzed by Type I Topoisomerases. BioMed Research International, 1–8.
-
Gurtovenko, A. A., & Anwar, J. (2007). Modulating the Structure and Properties of Cell Membranes: The Molecular Mechanism of Action of Dimethyl Sulfoxide. The Journal of Physical Chemistry B, 111(35), 10453–10460.
-
Shulyak, A., Goydyk, V., Gusakovsky, S., Grygorenko, V., Mytsyk, Y., & Badiuk, N. (2021). Modern aspects of the use of dimethyl sulfoxide (DMSO). Pharmacologyonline, 1, 82-89.